扩展功能
文章信息
- 段懿涵, 盛瑜, 徐健, 卢学春, 杜培革, 安丽萍
- DUAN Yihan, SHENG Yu, XU Jian, LU Xuechun, DU Peige, AN Liping
- 姬松茸多糖对D-半乳糖诱导的衰老模型小鼠的抗衰老作用及其Keap1/Nrf2/ARE信号转导途径机制
- Anti-aging effects of Agaricus blazei polysaccharide in D-galactose-induced aging model mice and Keap1/Nrf2/ARE signal transduction pathway mechanism
- 吉林大学学报(医学版), 2020, 46(02): 346-351
- Journal of Jilin University (Medicine Edition), 2020, 46(02): 346-351
- 10.13481/j.1671-587x.20200223
-
文章历史
- 收稿日期: 2019-12-05
衰老作为一种不可避免的生理过程,可导致身体部分功能逐渐丧失,其中认知能力下降已成为老年人最大的健康威胁之一[1]。近年来有许多关于衰老相关机制的研究,如衰老的端粒学说、自由基学说和代谢失衡学说等[2-4]。其中,自由基学说表明当人体内自由基生成过多或对其清除能力降低时,将发生各种严重病变[5],因此清除过量活性氧自由基具有重要的生理意义,安全有效的天然抗衰老药物成为人们关注焦点。
姬松茸(Agaricus blazei Murill, ABM)又名巴西蘑菇,是食药兼用的名贵真菌[6]。姬松茸子实体主要成分包括蛋白质、多糖、脂肪、纤维和维生素等,其中多糖含量可达45%。多糖不仅是维持生命活动的功能性大分子,同时有广泛的药理学作用,如降血脂、降血糖、抗血栓、保肝和通过增加白细胞含量减少放射性破坏的生物活性[7]。研究[8]表明:姬松茸多糖(Agaricus blazei polysaccharide, ABP)具有抗肿瘤、调节免疫、改善脂质水平和保护损伤神经等作用。目前,关于ABP抗衰老功效少有报道。本课题组前期研究[9]证实:ABP具有明显的抗氧化活性,体外清除自由基作用显著,由此本文作者推测ABP可能具有抗衰老的作用。
本研究分离纯化获得姬松茸酸性多糖A级分(Agaricus blazei polysaccharide-A,ABP-A),利用D-半乳糖(D-galactose, D-Gal)诱导建立小鼠衰老模型,观察ABP-A的抗衰老作用,探讨其抗衰老作用的分子机制,旨在为研发天然的抗衰老药物提供新资源和研究基础,为进一步开发和利用ABM提供实验依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器选取48只雄性ICR小鼠,体质量200~220 g,由长春亿斯实验动物技术有限公司提供,动物许可证号:SCXK(吉)2016-0003,本实验通过本校实验动物伦理委员会审查(伦理号IACUC-2018-004),实验过程遵循3R原则给予人道关怀。ABM(沈阳聚鑫北虫草菌业有限公司,由北华大学药学院药用植物学教研室韩冬老师鉴定合格),D-Gal(美国Genview公司),超氧化物歧化酶(superoxide dismutase,SOD)、丙二醛(malondialdehyde,MDA)、总抗氧化能力(total antioxidant capacity,T-AOC)、过氧化氢酶(catalase,CAT)和活性氧(reactive oxygen species,ROS)测试盒(南京建成生物研究所),一抗β-actin、Nrf2、Keap1、HO-1和二抗HRP-Goat Anti-Rabbit IgG(H+L)(美国ABclona公司)。Infinite M200型酶标仪(瑞士TECAN公司),SW-CJ-2D型双人净化工作台(上海苏净实业有限公司),TM-100 Morris水迷宫BA-200、小鼠避暗仪DT-200和小鼠跳台测试箱(成都泰盟技术有限责任公司),BA 400显微镜(麦克奥迪实业集团公司),离心机(德国Eppendorf公司),超微量核酸蛋白测定仪(德国Scandrop公司)。
1.2 ABP-A的制备取干燥至恒重的ABM,按水料比20:1(V/W),100℃提取3次,每次3 h,提取液浓缩离心,80%乙醇醇沉上清液,静置过夜,离心收集沉淀,依次用95%乙醇、无水乙醇洗涤,常规干燥得水提姬松茸粗多糖,透析,冷冻干燥,得ABP;利用DEAE-纤维素柱(7.5×30 cm,Cl-型)进行分级,0.5 mol·L-1NaCl洗脱,除盐,冷冻干燥,得到ABP-A。
1.3 实验动物分组和给药48只ICR小鼠随机分为4组,模型组小鼠颈背部皮下注射400 mg·kg-1·d-1D-Gal;对照组小鼠注射同剂量生理盐水;阳性药组小鼠先皮下注射400 mg·kg-1·d-1D-Gal,然后灌胃800 mg·kg-1吡拉西坦(按照人每日口服剂量为40~80 mg·kg-1);ABP-A组小鼠先皮下注射400 mg·kg-1·d-1D-Gal,然后灌胃400 mg·kg-1 ABP-A(按照人每日口服最大剂量计算)。连续给药10周。
1.4 行为学实验给药第9周进行避暗实验,将小鼠面部背对洞口放入明室中,进行训练和重实验,记录5 min内小鼠进入暗室的次数和首次进入暗室的时间,即错误次数和潜伏期,间隔24 h后开始实验。
跳台实验:小鼠遭电击后逃到安全台上,再次下台遭受电击时视为错误反应,记录3 min内的潜伏期和错误次数[10],24 h后再次测验。
Morris水迷宫定位航行实验:设定实验时间为120 s,小鼠从入水到找到安全平台所需的时间即为逃避潜伏期。若120 s内找到平台,则使其在平台上停留10 s;若120 s内未找到平台,则将其引导至平台,并停留10 s,潜伏期记录为120 s。第7天开始空间搜索,撤掉平台,将小鼠从第Ⅱ象限入水,持续时间120 s。记录小鼠进入平台、目标区域及目标象限的次数。
1.5 各组小鼠血清生化指标检测小鼠行为学实验结束后,将小鼠眼球取血,离心,取血清备用。处死的小鼠,迅速于冰台上取出全脑。严格按照各试剂盒说明书进行操作,对小鼠血清进行检测。采用酶联反应法分析ROS水平,黄嘌呤氧化酶法测定SOD活性,比色法检测T-AOC活性,采用比色法检测CAT活性及硫代巴比妥酸法测定MDA水平。
1.6 Western blotting法检测各组小鼠脑组织中Nrf2、Keap1和HO-1蛋白表达水平提取小鼠脑组织海马区蛋白,以12% SDS-PAGE凝胶电泳分离,转膜2 h至甲醇处理后的聚丙二氟乙烯膜(PVDF)上,转膜,置于摇床,用含5%脱脂奶粉的TBS-T封闭液封闭1 h,然后加入一抗β-actin(1:50 000)、Nrf2(1:500)、Keap1(1:500)和HO-1(1:500)室温孵育2 h,采用TBS-T洗5次,每次15 min,加入二抗(1:2 000)室温孵育1 h,TBS-T洗5次,每次15 min,最后加入ECL显色液显色。将显影得到的条带进行灰度分析,以β-actin为内参,以Keap1、Nrf2和HO-1各条带与内参条带灰度值的比值表示蛋白表达水平。
1.7 统计学分析采用SPSS 20.0统计软件进行统计学分析。各组小鼠避暗和跳台实验潜伏期、错误次数,水迷宫实验空间探索指标,各组小鼠血清中SOD和CAT活性及T-AOC、ROS和MDA水平,脑组织中Nrf2、Keap1和HO-1蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析,组间样本均数两两比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 ABP-A的制备透析袋透析除去灰分,得ABP,利用DEAE-纤维素柱进行离子交换层析,0.5 mol·L-1 NaCl溶液洗脱得到ABP-A。ABP得率为4.7%、总糖含量为75.1%、糖醛酸含量为1.9%、蛋白含量为5.6%;ABP-A得率为47.5%、总糖含量为74.1%、糖醛酸含量为8.2%。见表 1。
| (η/%) | |||||||||||||||||||||||||||||
| Polysaccharide | Yield | Total sugar | Uronic acid | protein | Ash | ||||||||||||||||||||||||
| ABP | 4.7 | 75.1 | 1.9 | 5.6 | 5.1 | ||||||||||||||||||||||||
| ABP-A | 47.5 | 74.1 | 8.2 | 4.8 | 5.1 | ||||||||||||||||||||||||
避暗实验结果显示:与对照组比较,模型组小鼠避暗潜伏期明显缩短(P < 0.05或P < 0.01),错误次数明显增多(P < 0.05);与模型组比较,阳性药物组和ABP-A组小鼠避暗潜伏期延长(P < 0.05或P < 0.01),错误次数减少(P < 0.05)。跳台实验结果显示:与对照组比较,模型组小鼠跳台潜伏期明显缩短(P < 0.05),错误次数明显增多(P < 0.05);与模型组比较,阳性药物组和ABP-A组小鼠跳台潜伏期延长(P < 0.05或P < 0.01),错误次数减少(P < 0.05)。见表 2。
| (n=12, x±s) | |||||||||||||||||||||||||||||
| Group | Dark avoidance latency(t/s) | Number of dark avoidance errors | Step down latency(t/s) | Number of platform errors | |||||||||||||||||||||||||
| Training | Retention | Training | Retention | Training | Retention | Training | Retention | ||||||||||||||||||||||
| Control | 24.54±2.50 | 90.60±2.84 | 5.56±0.51 | 3.94±0.84 | 29.39±2.50 | 99.58±2.84 | 2.86±0.22 | 1.00±0.20 | |||||||||||||||||||||
| Model | 18.49±1.61* | 34.29±3.91** | 8.94±0.25* | 7.82±0.71* | 13.09±3.61* | 76.49±4.91* | 3.33±0.31* | 1.93±0.26* | |||||||||||||||||||||
| Positive drug | 20.91±3.59 | 85.89±3.94△△ | 6.82±0.32△ | 4.65±0.44△ | 35.63±3.59△ | 86.10±3.94△ | 2.13±0.19△ | 1.40±0.27△ | |||||||||||||||||||||
| ABP-A | 14.88±2.53 | 95.84±3.96△△ | 6.06±0.34△ | 4.50±0.66△ | 32.03±2.53△ | 88.03±5.96△△ | 2.06±0.34△ | 1.31±0.21△ | |||||||||||||||||||||
| * P<0.05, * * P<0.01 compared with control group; △ P<0.05, △△ P<0.01 compared with model group. | |||||||||||||||||||||||||||||
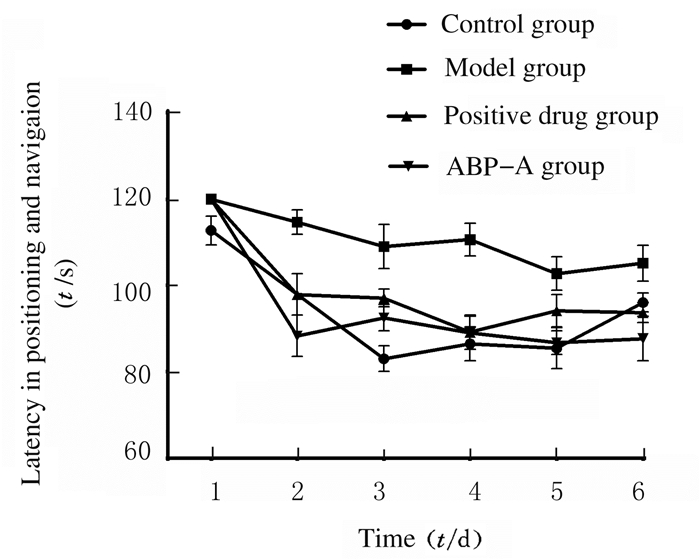
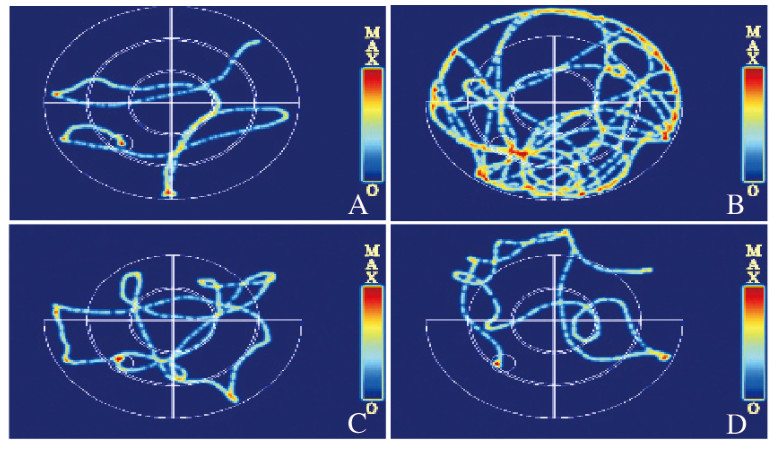
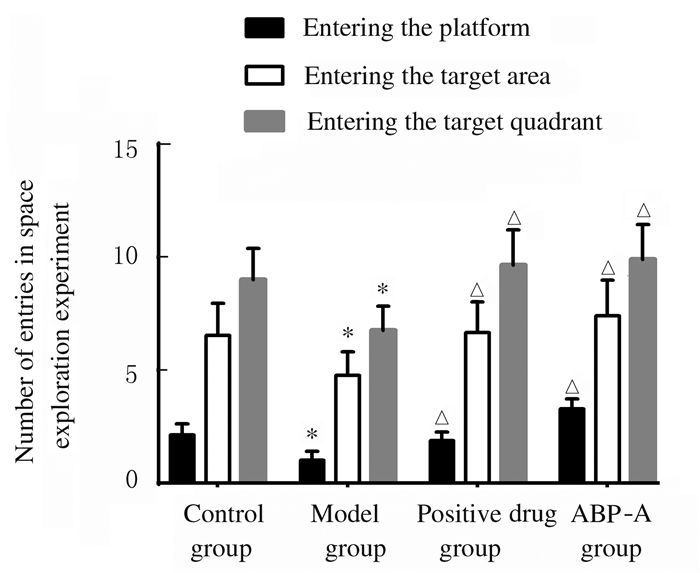
Morris水迷宫实验结果显示:定位航行实验中,随着训练时间的延长,各组小鼠定位航行潜伏期呈下降趋势(P < 0.05)。对照组小鼠定位航行潜伏期下降幅度最大,模型组下降幅度最小。第2~6天,与对照组比较,模型组小鼠定位航行潜伏期明显延长(P < 0.01);与模型组比较,阳性药物组和ABP-A组小鼠定位航行潜伏期从第2天开始明显缩短(P < 0.05)。见图 1。空间搜索实验中,与模型组比较,阳性药物组和ABP-A组小鼠进入平台的时间(潜伏期)明显缩短(P < 0.05)。进入平台的次数明显增加(P < 0.05)。见图 2(插页五)和图 3。
|
| 图 1 Morris水迷宫实验中各组小鼠定位航行潜伏期 Fig. 1 Latencies of positioning and navigation of mice in various groups in Morris water maze test |
|
|
|
| A: Control group; B: Model group; C: Positive drug group; D: ABP-A group. 图 2 各组小鼠定位航行实验典型热图分析 Fig. 2 Typical heat map analysis of positioning and navigation experiment of mice in various groups |
|
|
|
| *P < 0.05 compared with control group; △P < 0.05 compared with model group. 图 3 Morris水迷宫实验中各组小鼠空间探索指标 Fig. 3 Space exploration indicators of mice in various groups in Morris water maze test |
|
|
与对照组比较,模型组小鼠血清中SOD和CAT活性及T-AOC明显降低(P < 0.05),ROS水平升高(P < 0.05),MDA水平明显升高(P < 0.01);与模型组比较,阳性药物组和ABP-A组小鼠血清中SOD活性和T-AOC升高(P < 0.05),CAT活性明显升高(P < 0.05或P < 0.01),ROS水平降低(P < 0.05),MDA水平明显降低(P < 0.01)。见表 2。
| (n=12, x±s) | |||||||||||||||||||||||||||||
| Group | SOD [λB/(U·mL-1)] |
CAT [λB/(U·mg-1)] |
T-AOC [cB/(mmol·L-1)] |
ROS [λB/(U·mL-1)] |
MDA [cB/(nmol·L-1)] |
||||||||||||||||||||||||
| Control | 60.97±3.61 | 2.47±0.49 | 0.67±0.08 | 60.77±5.65 | 1.69±0.52 | ||||||||||||||||||||||||
| Model | 46.55±5.52* | 2.06±0.37* | 0.56±0.04* | 84.67±7.59* | 4.82±0.24** | ||||||||||||||||||||||||
| Positive drug | 62.08±2.76△ | 2.57±0.11△ | 0.70±0.06△△ | 75.29±9.45△ | 1.33±0.34△△ | ||||||||||||||||||||||||
| ABP-A | 60.93±3.64△ | 3.73±0.48△△ | 0.65±0.04△ | 70.93±9.90△ | 1.57±0.29△△ | ||||||||||||||||||||||||
| * P<0.05, * * P<0.01 compared with control group; △ P<0.05, △△ P<0.01 compared with model group. | |||||||||||||||||||||||||||||
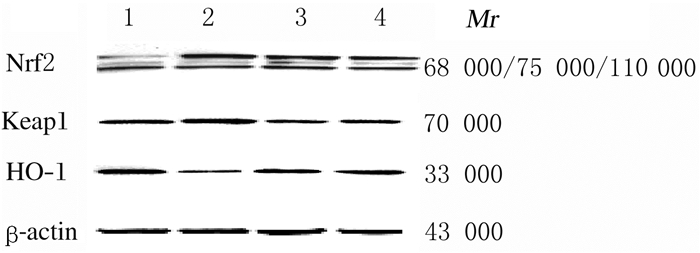
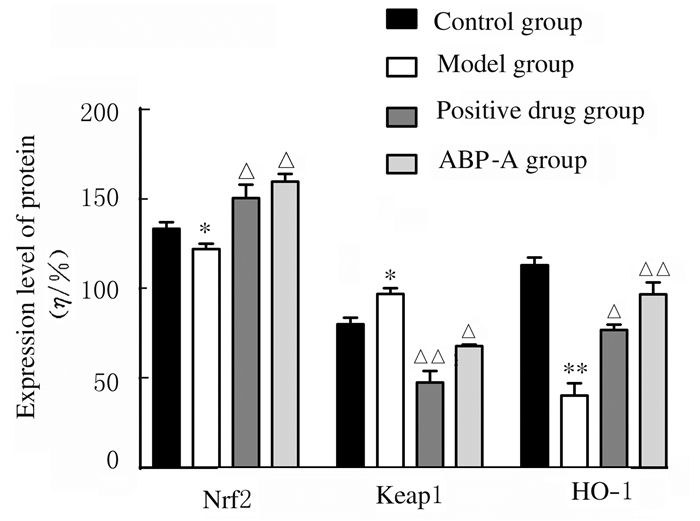
与对照组比较,模型组小鼠脑组织中Nrf2和HO-1蛋白表达水平均明显降低(P<0.05或P<0.01),Keap1蛋白表达水平升高(P<0.05);与模型组比较,阳性药物组和ABP-A组小鼠脑组织中Keap1蛋白表达水平明显降低(P<0.05或P<0.01),Nrf2和HO-1蛋白表达水平明显升高(P<0.05或P<0.01)。见图 4和图 5。
|
| Lane1: Control group; Lane2:Model group; Lane3:Positive drug group; Lane4:ABP-A group. 图 4 Western blotting法检测各组小鼠脑组织中Nrf2、Keap1和HO-1蛋白表达电泳图 Fig. 4 Electrophoregram of expressions ofNrf2, Keap1 and HO-1 proteins in brain tissue of mice in various groups detected by Western blotting method |
|
|
|
| *P < 0.05, * *P < 0.01 compared with control group; △P < 0.05, △△P < 0.01 compared with model group. 图 5 各组小鼠脑组织中Nrf2、Keap1和HO-1蛋白表达水平 Fig. 5 Expression levels of Nrf2, Keap1 and HO-1 proteins in brain tissue of mice in various groups |
|
|
衰老是生命发展的最后一个阶段,是随着时间的推移,机体的各种功能不断下降的过程, 衰老的发生与氧化应激的积累密切相关[11]。D-Gal作为生理性营养成分,过量时会诱发机体氧化损伤、炎症以及细胞凋亡等, 与自然衰老症状相似[12-13]。本研究中连续给予过量D-Gal后,模型组小鼠活动减少,毛发脱落,行为学实验表现出潜伏期较短,错误次数增多现象,说明小鼠衰老模型建立成功。SOD和CAT等组成的酶系统具有内源性抗氧化损伤作用,通过代谢转化可去除过量ROS减轻氧化应激[14]。T-AOC通常被认为是体内所有抗氧化剂的累积作用,具有评估抗氧化能力的作用[15]。MDA是氧自由基和脂质氧化的降解产物,其水平的升高导致自由基的产生增多和(或)抗氧化物质功能下降[16]。本研究结果显示:给予ABP-A干预后,小鼠血清中SOD和CAT活性升高,T-AOC升高,MDA和ROS水平降低,小鼠一般状态得到有效改善,学习记忆能力提高,说明ABP-A能减缓由D-Gal诱导的机体氧化损伤程度,进而对衰老引起的学习记忆衰退具有良好的修复作用。
Keap1/Nrf2/ARE信号通路的激活可诱导产生一系列内源性酶,如SOD、CAT、谷胱甘肽过氧化物酶和过氧还原酶等,这些自由基清除酶能够参与机体的抗氧化防御机制[17]。此外,Keap1/Nrf2/ARE作为氧化应激中重要的信号通路之一,是防御外源物质和细胞氧化损伤,减少氧化应激和介导抗氧化的主要途径[18-19],其中,Keap1蛋白含有半胱氨酸残基,这些残基在维持细胞氧化还原平衡方面起着至关重要的作用;转录因子Nrf2是抵御细胞环境压力的核心,在氧化应激中具有中心地位。在正常情况下,Keap1与Nrf2结合锚定在细胞质内,使Nrf2处于非活性状态。而在氧化应激下,Nrf2与其负调控因子Keapl脱离,并从细胞质转移至细胞核中[20]。Nrf2在细胞核内调节与DNA上的抗氧化反应元件ARE结合,上调可以催化体内多种抗氧化途径的下游基因HO-1的转录和表达[21], 清除体内过量ROS抵抗氧化应激, 从而起到细胞保护作用。本研究结果显示:ABP-A干预后,模型小鼠脑组织中Keap1表达水平下调,且Nrf2和HO-1表达水平上调,表明ABP-A抗衰老作用与激活Keap1/Nrf2/ARE信号通路有关联。
综上所述,ABP-A可以改善D-Gal诱导的衰老过程中的小鼠脑损伤,提高机体内的抗氧化酶活性,并降低脂质过氧化,具有明显的抗衰老作用,其抗衰活性可能与激活Keap1/Nrf2/ARE信号转导途径有关。本研究为ABM作为保健品和药品原料的研究提供了理论依据,对促进ABM的产业化深度开发具有重要意义。
| [1] |
LI H D, ZHAO H J, GAO Z, et al. Pleurotus djamor the antioxidant and anti-aging effects of acetylated mycelia polysaccharides from[J]. Molecules, 2019, 24(15): 2698. DOI:10.3390/molecules24152698 |
| [2] |
UR R S, ALI S S, TAHIR A, et al. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats[J]. Mol Neurobiol, 2017, 54: 255-271. DOI:10.1007/s12035-015-9604-5 |
| [3] |
SFEIR A J, CHAI W, SHAY J W. Telomereend processing the terminal nucleotides of human chromosomes[J]. Mol Cell, 2005, 18(1): 131-138. DOI:10.1016/j.molcel.2005.02.035 |
| [4] |
FRAUKE B, SHAHRZAD K M, JURGEN K, et al. A metabolic Obesity Profile is associated with decreased gray matter volume in cognitively healthy older adults[J]. Front Aging Neurosci, 2019, 11: 202. DOI:10.3389/fnagi.2019.00202 |
| [5] |
DRUZIAN S P, PINHEIRO L N, SUSIN N M, et al. Production of metabolites with antioxidant activity by Botryosphaeria dothidea in submerged fermentation[J]. Bioprocess Biosystems Engineer, 2020, 43(1): 13-20. DOI:10.1007/s00449-019-02200-y |
| [6] |
VEERAPPAN V G, MUTHU R, et al. Agaricus blazei extract abrogates rotenone-induced dopamine depletion and motor deficits by its anti-oxidative and anti-inflammatory properties in Parkinsonic mice[J]. Nutr Neurosci, 2018, 21: 657-666. DOI:10.1080/1028415X.2017.1337290 |
| [7] |
王夏梅.姬松茸子实体多糖分离纯化、结构表征和免疫活性分析[D].无锡: 江南大学, 2016.
|
| [8] |
JIANG L Y, YU Z P, LIN Y, et al. Agaricus blazeiLow-molecular-weight polysaccharides from Murrill modulate the Th1 response in cancer immunity[J]. Oncol Lett, 2018, 15: 3429-3436. |
| [9] |
安丽萍, 段懿涵, 盛瑜, 等. 姬松茸多糖酶降解对D-半乳糖诱导3T3细胞氧化损伤的保护作用[J]. 北华大学学报(自然科学版), 2019, 20(5): 653-658. |
| [10] |
ZHAO S S, YANG W, JIN H, et al. Puerarin attenuates learning and memory impairments and inhibits oxidative stress in STZ-induced SAD mice[J]. Neurotoxicology, 2015, 51: 166-171. DOI:10.1016/j.neuro.2015.10.010 |
| [11] |
ZHANG H H, ZHENG W, FENG X L, et al. Nrf2/ARE signaling Acts as master pathway for the cellular antioxidant activity of fisetin[J]. Molecules, 2019, 24(4): 708. DOI:10.3390/molecules24040708 |
| [12] |
SADIGH E S, MAJDI A, MCCANN S K, et al. D-galactose-induced brain ageing model:A systematic review and meta-analysis on cognitive outcomes and oxidative stress indices[J]. PLoS One, 2017, 12(8): e0184122. DOI:10.1371/journal.pone.0184122 |
| [13] |
ISLAM M T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders[J]. Neurol Res, 2017, 39(1): 73-82. DOI:10.1080/01616412.2016.1251711 |
| [14] |
SUN X, SUN G B, WANG M, et al. Protective effects of cynaroside against H2O2induced apoptosis in H9c2 cardiomyoblasts[J]. J Cell Biochem, 2011, 112(8): 2019-2029. DOI:10.1002/jcb.23121 |
| [15] |
HE H B, XU J, XU Y Q, et al. Cardioprotective effects of saponins from Panax japonicus on acute myocardial ischemia against oxidative stresstriggered damage and cardiac cell death in rats[J]. J Ethnopharmacol, 2012, 140(1): 73-82. DOI:10.1016/j.jep.2011.12.024 |
| [16] |
SINGH C K, CHHABRA G, NDIAYE M, et al. The role of sirtuins in antioxidant and redox signaling[J]. Antioxid Redox Signal, 2018, 28(8): 643-661. DOI:10.1089/ars.2017.7290 |
| [17] |
WANG Z, JI C Y, WU L Y, et al. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage:role of Keap1/Nrf2/ARE pathway[J]. PLoS One, 2014, 9: e97685. DOI:10.1371/journal.pone.0097685 |
| [18] |
KUBBEN N, ZHANG W, WANG L, et al. Repression of the antioxidant NRF2 pathway in premature aging[J]. Cell, 2016, 165(6): 1361-1374. DOI:10.1016/j.cell.2016.05.017 |
| [19] |
MAHMOUD A M, GERMOUSH M O, ALOTAIBI M F, et al. Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity[J]. Biomed Pharmacother, 2016, 86: 297-306. |
| [20] |
ZHANG L, WANG H D, FAN Y W, et al. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways[J]. Sci Rep, 2017, 7: 46763. DOI:10.1038/srep46763 |
| [21] |
YA B L, LIU Q, LI H F, et al. Uric acid protects against focal cerebral ischemia/reperfusion-induced oxidative stress via activating Nrf2 and regulating neurotrophic factor expression[J]. Oxid Med Cell Longev, 2018, 2018: 6069150. |
 2020, Vol. 46
2020, Vol. 46


