扩展功能
文章信息
- 苏龙, 张云蔚, 王卓, 宋飞, 秦田雪, 高素君
- SU Long, ZHANG Yunwei, WANG Zhuo, SONG Fei, QIN Tianxue, GAO Sujun
- 紫草素对人白血病MV4-11细胞的增殖抑制和促凋亡作用
- Proliferation inhibition and pro-apoptotic effects of shikonin on human leukemia MV4-11 cells
- 吉林大学学报(医学版), 2020, 46(01): 96-101
- Journal of Jilin University (Medicine Edition), 2020, 46(01): 96-101
- 10.13481/j.1671-587x.20200117
-
文章历史
- 收稿日期: 2019-10-15
急性髓系白血病(acute myeloid leukemia, AML)患者FMS样酪氨酸激酶3受体基因内部串联重复序列(FMS-like tyrosine kinase-3 receptorinternal tandem duplication, FLT3-ITD)突变的发生率为20%~30%,FLT3-ITD突变AML危险分层属于高危组,预后不良[1-3]。微小RNA(microRNAs)在髓细胞分化和白血病发生中均发挥十分重要的作用,microRNA-155(miR-155)是AML的不良预后指标之一[4]。在FLT3-ITD突变AML患者中,miR-155表达明显上调[5]。有文献[6]报道:通过靶向药物干扰miR-155表达,可诱导白血病细胞凋亡且能够延长白血病小鼠的生存期。miR-155可能是FLT3-ITD突变AML的理想治疗靶点之一。尽管紫草素的抗肿瘤作用在实体肿瘤中被广泛报道,但有关其抗白血病作用的研究[7-9]较少。紫草素对FLT3-ITD突变AML是否有作用目前尚不清楚,且紫草素对miR-155表达是否有影响亦无文献报道。本研究采用人FLT3-ITD突变AMLMV4-11细胞为研究对象,探讨紫草素对其增殖抑制和促凋亡作用,初步阐明其分子机制。
1 材料与方法 1.1 细胞、主要试剂和仪器MV4-11细胞系购自中国科学院(上海)细胞库,培养于含10%胎牛血清(美国Gemini公司)和1%青霉素-链霉素的DMEM培养基(美国赛默飞世尔公司)中。紫草素购自上海纯优生物有限公司,采用二甲基亚砜(DMSO)溶解,配制成10 g·L-1储存液备用。CCK-8试剂盒(上海东仁化学科技有限公司),Annexin Ⅴ-FITC/7-AAD试剂盒(美国BD公司),RNAiso Plus试剂盒[宝生物(大连)有限公司],miRcute miRNA提取分离试剂盒[天根生化科技(北京)有限公司],Real-time PCR试剂盒(美国赛默飞世尔公司)。流式细胞仪(美国BD公司),酶标仪(美国BioTek公司),PCR仪(美国Bio-Rad公司)。
1.2 CCK-8法检测细胞增殖抑制率采用CCK-8法检测细胞增殖抑制率,具体操作参照试剂盒说明书进行。取生长状态良好的MV4-11细胞,在96孔板中配制100 μL的细胞悬液(调整细胞密度为5×104 mL-1)。MV4-11细胞经不同浓度(0.5、1.0、2.0、4.0和8.0 μmol·L-1)紫草素处理24和48 h,同时设不加紫草素的DMSO组作为对照,每孔加入10 μL CCK-8溶液,将培养板在培养箱内孵育1.5 h,用酶标仪测定450 nm处的吸光度(A)值。每组设3个平行孔,实验重复3次。计算24和48 h时紫草素的半数抑制浓度(IC50)。按公式计算细胞增殖抑制率:细胞增殖抑制率= [1-(实验孔A值-空白孔A值)/(对照孔A值-空白孔A值)] × 100%。
1.3 羟基荧光素二醋酸盐琥珀酰亚胺脂(CSFE)标记法检测细胞增殖率取生长状态良好的MV4-11细胞,采用CFSE预孵育8 min。在96孔板中配制100 μL的细胞悬液(调整细胞密度为5×104mL-1)。MV4-11细胞经不同浓度(0.25、0.50和1.00 μmol·L-1)紫草素处理48和72 h,同时设空白对照组和DMSO组。采用流式细胞仪检测各组CFSE标记细胞百分率,即细胞增殖率。
1.4 流式细胞术检测细胞凋亡率采用不同浓度(1/2×IC50、IC50和2×IC50)紫草素处理MV4-11细胞24~48 h,同时设不加紫草素的DMSO组作为对照。收集用于凋亡检测的细胞,1 000 r·min-1离心5 min,去除培养基,PBS冲洗2次,加入Anexin Ⅴ-FITC 5 μL和7-AAD5 μL,轻柔弹起混匀,4℃避光孵育30 min。1h内采用流式细胞仪检测各组细胞凋亡率。实验重复3次。
1.5 Real-time PCR法检测细胞中miR-155表达水平MV4-11细胞分为DMSO组和不同浓度(1/4×IC50、1/2×IC50和IC50)紫草素组,处理48 h后,采用RNAiso Plus试剂盒和miRcute miRNA提取分离试剂盒提取总RNA,具体操作参照说明书。采用逆转录试剂盒进行反转录,采用Real-time PCR试剂盒检测基因表达,以2-ΔΔCt法计算miR-155的相对表达水平,实验重复3次。相关引物序列如下:Human U6,F 5′-CTCGCTTCGGCAGCACA-3′,R5′-AACGCTTCACGAA- TTTGCGT-3′;miR-155,F5′-TTAATGCTAATCGTGATAGGGGT-3′,R5′-GCTGTCAACG- ATACGCTACCTA-3′。
1.6 统计学分析采用SPSS 17.0统计软件进行统计学分析。各组细胞增殖抑制率、凋亡率和细胞中miR-155表达水平均符合正态分布且总体方差齐,以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用LSD检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组细胞增殖抑制率和增殖率采用不同浓度紫草素(0.5~8.0 μmol·L-1)处理MV4-11细胞24~48 h,CCK-8法检测各组细胞增殖抑制率,结果显示:与DMSO组比较,不同浓度紫草素组MV4-11细胞的增殖抑制率明显升高(P < 0.05或P < 0.01),且随着紫草素浓度的升高各组细胞增殖抑制率逐渐升高(表 1)。根据不同浓度紫草素组细胞的增殖抑制率结果计算,24和48 h时紫草素的IC50分别为1.743 (1.468~2.118) μmol·L-1和1.404 (1.228~1.612) μmol·L-1。不同浓度紫草素(0.25、0.50和1.00 μmol·L-1)处理MV4-11细胞48和72 h,采用CFSE标记法检测各组细胞增殖率,结果显示:与空白对照组和DMSO组比较,不同浓度紫草素组细胞增殖率均明显降低。见图 1。
| (n=3, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | Inhibitory rate of proliferation | ||||||||||||||||||||||||||||
| (t/h) 24 | 48 | ||||||||||||||||||||||||||||
| DMSO | 1.47±0.91 | 2.67±1.48 | |||||||||||||||||||||||||||
| Shikonin (μmol·L-1) | |||||||||||||||||||||||||||||
| 0.5 | 9.13±3.21* | 10.83±4.05* | |||||||||||||||||||||||||||
| 1.0 | 19.17±2.73** | 29.40±3.50** | |||||||||||||||||||||||||||
| 2.0 | 36.07±6.07** | 60.70±5.07** | |||||||||||||||||||||||||||
| 4.0 | 79.43±4.83** | 86.60±5.15** | |||||||||||||||||||||||||||
| 8.0 | 88.07±2.41** | 91.67±1.74** | |||||||||||||||||||||||||||
| * P < 0.05, * * P < 0.01 compared with DMSO group. | |||||||||||||||||||||||||||||
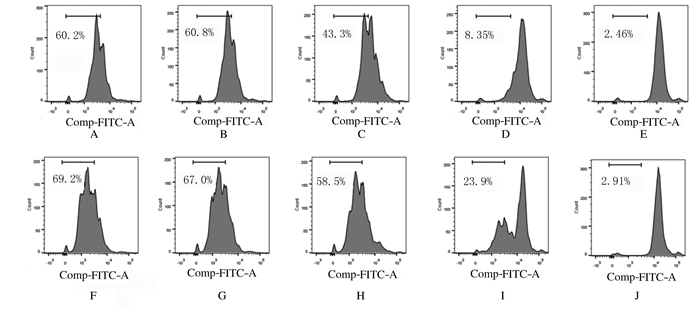
|
| A-E: 48 h; F-J: 72 h; A, F: Blank control group; B, G: DMSO group; C, H: 0.25 μmol·L-1 shikonin group; D, I: 0.50 μmol·L-1 shikonin group; E, J: 1.00 μmol·L-1 shikonin group. 图 1 CFSE法检测各组MV4-11细胞增殖率 Fig. 1 Proliferation rates of MV4-11 cells in various groups determined by CFSE method |
|
|
作用48 h时紫草素的IC50为1.404 μmol·L-1。MV4-11细胞经不同浓度[0.702(1/2×IC50)、1.404(IC50)和2.808 μmol·L-1(2×IC50)]紫草素处理48 h,采用流式细胞术检测细胞凋亡率,结果显示:DMSO组和紫草素0.702、1.404及2.808 μmol·L-1紫草素组细胞凋亡率分别为(7.96 ± 1.39)%、(11.33 ± 4.28)%、(21.02 ± 3.59)%和(23.65 ± 2.65)%,与DMSO组比较,不同浓度紫草素组细胞凋亡率明显升高(P < 0.01),且呈剂量依赖性。见图 2。
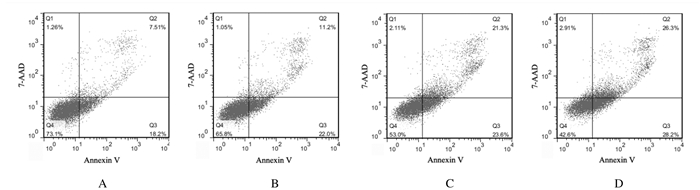
|
| A: DMSO group; B: 0.702 μmol·L-1 shikonin group; C: 1.404 μmol·L-1 shikonin group; D: 2.808 μmol·L-1 shikonin group. 图 2 不同浓度紫草素作用48 h时各组MV4-11细胞凋亡率 Fig. 2 Apoptotic rates of MV4-11 cells in various groups after treated with different concentrations of shikonin for 48 h |
|
|
不同浓度紫草素处理MV4-11细胞48 h,采用Real-time PCR法检测各组细胞中miR-155表达水平,结果显示:与DMSO组比较,0.702和1.404 μmol·L-1紫草素组细胞中miR-155表达水平明显降低(P < 0.01);1.404 μmol·L-1紫草素组细胞中miR-155表达水平降低超过75%。见图 3。
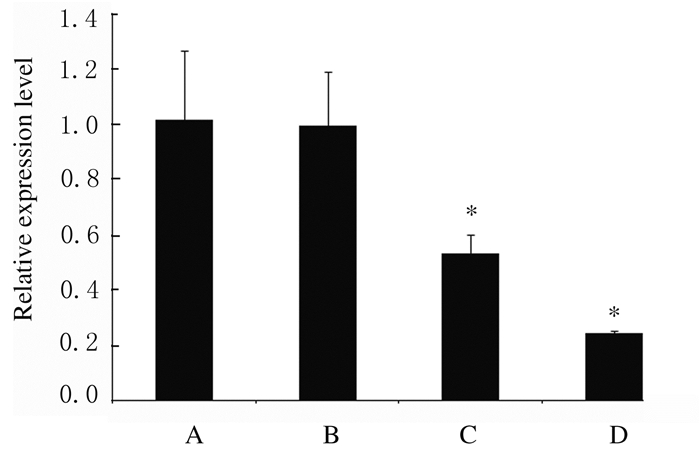
|
| *P < 0.01 compared with DMSO group.A: DMSO group; B: 0.351 μmol·L-1 shikonin group; C: 0.702 μmol·L-1 shikonin group; D: 1.404 μmol·L-1 shikonin group. 图 3 不同浓度紫草素作用48 h时各组MV4-11细胞中miR-155表达水平 Fig. 3 Expression levels of miR-155 in MV4-11 cells in various groups after treated with different concentrations of shikonin for 48 h |
|
|
FLT3-ITD突变是AML最常见的基因突变,为AML的不良预后分子标志之一。与野生型患者比较,FLT3-ITD突变患者具有骨髓原始细胞高、外周血白细胞高、初始诱导治疗缓解率低、早期死亡率高和复发风险高等特点,采用联合化疗患者长期存活率仅为20%~30%[10-12]。异基因造血干细胞移植(allogeneic hematopoietic stem cell transplantation, allo-HSCT)是目前唯一有望彻底治愈FLT3-ITD突变AML患者的治疗手段。多项研究[13-14]显示:FLT3-ITD突变是接受allo-HSCT患者的不良预后指标之一,与野生型患者比较,FLT3-ITD突变患者allo-HSCT后复发率更高且存活期更短。但也有研究[15-17]报道:FLT3-ITD突变对allo-HSCT后患者预后无明显影响。上述研究结果的差异可能与预处理方案、移植类型和移植后抢先干预手段等不同有关。因此,allo-HSCT是否可逆转FLT3-ITD突变AML患者的不良预后尚存在一定争议。近年来靶向药物在治疗FLT3-ITD突变AML患者治疗中取得一定进展[18]。2017年《新英格兰杂志》报道了应用米哚托林治疗FLT3-ITD突变AML患者临床试验的结果,结果显示:米哚托林组患者4年的无疾病生存率和总生存率分别为51.4%和28.2%,安慰剂组为44.3%和20.6%[19]。尽管患者可以从米哚托林治疗中获益,该药也已被美国食品药品监督管理局(FDA)批准用于治疗FLT3-ITD突变AML患者,但目前国内患者无法使用且疗效改善仍有限。FLT3-ITD突变AML患者的治疗仍有待改善。
FLT3-ITD突变可导致受体持续活化,进而激活一系列下游信号分子,如核因子-κB(nuclearfactor-κB, NF-κB),后者可通过结合miR-155的启动子进而上调miR-155表达;miR-155可抑制SHIP1、PU.1和CCAAT增强子结合蛋白A(CEBPA)表达,SHIP1是磷脂酰肌醇3-激酶(PI3K)/蛋白激酶B(AKT)通路的抑制物,而PU.1和CEBPA是髓细胞分化过程中十分重要的转录因子。因此,FLT3-ITD突变可导致造血细胞分化受阻并大量增殖,并最终形成急性白血病[5]。多项研究[4, 20-21]表明:FLT3-ITD突变AML患者miR-155表达水平明显升高,且为AML的不良预后因素之一。动物实验[6]表明:抑制白血病小鼠miR-155表达可明显延长小鼠的生存期。因此,调控miR-155可能是治疗FLT3-ITD突变患者的重要策略之一。
已有研究[7-9]报道:紫草素具有抗白血病效应。紫草素可通过含半胱氨酸的天冬氨酸蛋白水解酶3(caspase-3)途径诱导人类急性早幼粒细胞白血病HL60细胞系凋亡[7]。在BCR-ABL阳性慢性粒细胞白血病细胞中,紫草素可通过活性氧(reactive oxygen species, ROS)/ c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)途径诱导细胞凋亡[8]。近来有研究[9]报道了紫草素杀伤U937白血病细胞的新机制,紫草素可能通过细胞外调节蛋白激酶(ERK)/JNK/丝裂原活化蛋白激酶(MAPK)和AKT通路抑制c-MYC基因进而诱导细胞凋亡。然而,紫草素对人FLT3-ITD突变AML细胞系是否有作用目前并不清楚。本研究结果显示:紫草素对MV4-11细胞系具有明显的增殖抑制作用和促凋亡作用,紫草素可明显下调MV4-11细胞中miR-155的表达水平。NF-κB通过结合miR-155启动子进而调节miR-155表达[15]。既往研究[22-23]报道:紫草素通过NF-κB发挥其主要生物学功能。因此,本文作者推测:紫草素可能通过抑制NF-κB入核进而下调miR-155的表达。此外,紫草素是否对miR-155下游信号分子有作用、是否可促进白血病细胞分化等尚有待进一步研究证实。
紫草素治疗AML具有一定优势。AML患者处于免疫低下状态,加之化疗,使患者的免疫力再次受到打击,细菌和真菌感染的发生率高,而紫草素具有一定的抗细菌和抗真菌感染能力[24]。关于药物安全性问题,本课题组的前期研究[25]并未发现紫草素及其衍生物具有明显的急性及慢性毒性反应:急性毒性实验采用小鼠模型,最大给药剂量为1 g·kg-1;慢性毒性实验采用大鼠模型,最高剂量为800 mg·kg-1灌胃,连续6个月。
综上所述,紫草素可抑制FLT3-ITD突变人白血病MV4-11细胞的增殖,促进细胞凋亡。此外,紫草素可下调AML不良预后分子miR-155的表达,进而有可能逆转或部分逆转FLT3-ITD突变AML患者的不良预后。因此,紫草素可作为FLT3-ITD突变AML的潜在治疗药物之一。
| [1] |
PATEL J P, GÖNEN M, FIGUEROA M E, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia[J]. N Engl J Med, 2012, 366(12): 1079-1089. DOI:10.1056/NEJMoa1112304 |
| [2] |
PAPAEMMANUIL E, GERSTUNG M, BULLINGER L, et al. Genomic classification and prognosis in acute myeloid leukemia[J]. N Engl J Med, 2016, 374(23): 2209-2221. DOI:10.1056/NEJMoa1516192 |
| [3] |
PASIC I, DA'NA W, LAM W, et al. Influence of FLT3-ITD and NPM1 status on allogeneic hematopoietic cell transplant outcomes in patients with cytogenetically normal AML[J]. Eur J Haematol, 2019, 102(4): 368-374. DOI:10.1111/ejh.13216 |
| [4] |
MARCUCCI G, MAHARRY K S, METZELER K H, et al. Clinical role of microRNAs in cytogenetically normal acute myeloid leukemia:miR-155 upregulation independently identifies high-risk patients[J]. J Clin Oncol, 2013, 31(17): 2086-2093. DOI:10.1200/JCO.2012.45.6228 |
| [5] |
GERLOFF D, GRUNDLER R, WURM A A, et al. NF-κB/STAT5/miR-155 network targets PU.1 in FLT3-ITD-driven acute myeloid leukemia[J]. Leukemia, 2015, 29(3): 535-547. DOI:10.1038/leu.2014.231 |
| [6] |
KHALIFE J, RADOMSKA H S, SANTHANAM R, et al. Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924(Pevonedistat) in FLT3-ITD acute myeloid leukemia[J]. Leukemia, 2015, 29(10): 1981-1992. DOI:10.1038/leu.2015.106 |
| [7] |
YOON Y, KIM YO, LIM NY, et al. Shikonin, an ingredient of Lithospermum erythrorhizon induced apoptosis in HL60 human premyelocytic leukemia cell line[J]. Planta Med, 1999, 65(6): 532-535. DOI:10.1055/s-1999-14010 |
| [8] |
MAO X, YU C R, LI W H, et al. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells[J]. Cell Res, 2008, 18(8): 879-888. DOI:10.1038/cr.2008.86 |
| [9] |
ZHAO Q L, ASSIMOPOULOU A N, KLAUCK S M, et al. Inhibition of c-MYC with involvement of ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and its derivatives in killing leukemia cells[J]. Oncotarget, 2015, 6(36): 38934-38951. |
| [10] |
KOTTARIDIS P D, GALE R E, FREW M E, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy:analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials[J]. Blood, 2001, 98(6): 1752-1759. DOI:10.1182/blood.V98.6.1752 |
| [11] |
THIEDE C, STEUDEL C, MOHR B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia:association with FAB subtypes and identification of subgroups with poor prognosis[J]. Blood, 2002, 99(12): 4326-4335. DOI:10.1182/blood.V99.12.4326 |
| [12] |
FRÖHLING S, SCHLENK R F, BREITRUCK J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics:A study of the AML Study Group Ulm[J]. Blood, 2002, 100(13): 4372-4380. DOI:10.1182/blood-2002-05-1440 |
| [13] |
SCHMID C, LABOPIN M, SOCIÉ G, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation[J]. Blood, 2015, 126(17): 2062-2069. DOI:10.1182/blood-2015-06-651562 |
| [14] |
SONG Y, MAGENAU J, LI Y M, et al. FLT3 mutational status is an independent risk factor for adverse outcomes after allogeneic transplantation in AML[J]. Bone Marrow Transplant, 2016, 51(4): 511-520. DOI:10.1038/bmt.2015.170 |
| [15] |
DEOL A, SENGSAYADETH S, AHN K W, et al. Does FLT3 mutation impact survival after hematopoietic stem cell transplantation for acute myeloid leukemia? A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis[J]. Cancer, 2016, 122(19): 3005-3014. DOI:10.1002/cncr.30140 |
| [16] |
CANAANI J, LABOPIN M, HUANG X J, et al. T-cell replete haploidentical stem cell transplantation attenuates the prognostic impact of FLT3-ITD in acute myeloid leukemia:A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation[J]. Am J Hematol, 2018, 93(6): 736-744. DOI:10.1002/ajh.25082 |
| [17] |
ZHANG Y Y, MO X D, ZHANG X H, et al. FLT3 internal tandem duplication does not impact prognosis after haploidentical allogeneic hematopoietic stem cell transplantation in AML patients[J]. Bone Marrow Transplant, 2019, 54(9): 1462-1470. DOI:10.1038/s41409-019-0456-x |
| [18] |
LARROSA-GARCIA M, BAER M R. FLT3 inhibitors in acute myeloid leukemia:current status and future directions[J]. Mol Cancer Ther, 2017, 16(6): 991-1001. DOI:10.1158/1535-7163.MCT-16-0876 |
| [19] |
STONE R M, MANDREKAR S J, SANFORD B L, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation[J]. N Engl J Med, 2017, 377(5): 454-464. DOI:10.1056/NEJMoa1614359 |
| [20] |
XU L H, GUO Y, CEN J N, et al. Overexpressed miR-155 is associated with initial presentation and poor outcome in Chinese pediatric acute myeloid leukemia[J]. Eur Rev Med Pharmacol Sci, 2015, 19(24): 4841-4850. |
| [21] |
RAMAMURTHY R, HUGHES M, MORRIS V, et al. MiR-155 expression and correlation with clinical outcome in pediatric AML:A report from Children's Oncology Group[J]. Pediatr Blood Cancer, 2016, 63(12): 2096-2103. DOI:10.1002/pbc.26157 |
| [22] |
ANDUJAR I, RECIO M C, BACELLI T, et al. Shikonin reduces oedema induced by phorbol ester by interfering with IkappaBalpha degradation thus inhibiting translocation of NF-kappaB to the nucleus[J]. Br J Pharmacol, 2010, 160(2): 376-388. DOI:10.1111/j.1476-5381.2010.00696.x |
| [23] |
CHENG Y W, CHANG C Y, LIN K L, et al. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-kappaB signaling[J]. J Ethnopharmacol, 2008, 120(2): 264-271. DOI:10.1016/j.jep.2008.09.002 |
| [24] |
ANDUJAR I, RÍOS J L, GINER R M, et al. Pharmacological properties of shikonin-a review of literature since 2002[J]. Planta Med, 2013, 79(18): 1685-1697. DOI:10.1055/s-0033-1350934 |
| [25] |
SU L, LIU L H, WANG Y L, et al. Long-term systemic toxicity of shikonin derivatives in Wistar rats[J]. Pharm Biol, 2013, 52(4): 486-490. |
 2020, Vol. 46
2020, Vol. 46


