扩展功能
文章信息
- 万琦, 余宝刚
- WAN Qi, YU Baogang
- miR-125b对大鼠心肌梗死后心室重构的调控作用
- Regulatory effect of miR-125b on ventricular remodeling after myocardial infarction in rats
- 吉林大学学报(医学版), 2020, 46(01): 84-89
- Journal of Jilin University (Medicine Edition), 2020, 46(01): 84-89
- 10.13481/j.1671-587x.20200115
-
文章历史
- 收稿日期: 2019-03-11
心室重构的病理基础为:在心肌梗死区,坏死心肌细胞由瘢痕组织取代[1];在非心肌梗死区,心肌细胞发生凋亡,细胞外基质网络结构重建,导致心脏功能和结构异常,进一步发展为心力衰竭,严重影响患者的生命健康。因此探讨心肌梗死后心室重构的机制对其诊治具有重要意义[2]。研究[3-4]显示:微小RNA-125b(micro RNA-125b,miR-125b)在心肌纤维化中发挥重要作用,抑制miR-125b可抑制心肌梗死后心室重构,但其具体机制尚不十分清楚。研究[5-6]表明:miR-125b可通过磷脂酰肌醇3激酶(phosphatidylionsitol 3-kinase,PI3K)/蛋白激酶B(protein kinases,Akt)等多种信号通路参与疾病的发生发展过程,PI3K/Akt信号通路的激活对心肌组织有保护作用。本研究拟探讨miR-125b通过PI3K/Akt信号通路对大鼠心肌梗死后心室重构的影响, 阐明其作用机制。
1 材料与方法 1.1 实验动物、主要试剂和仪器健康、清洁级SD大鼠75只,12周龄,雌雄各半,体质量200~250 g,购自北京华阜康生物科技股份有限公司,动物许可证号:SCXK(京)2014-0008。miR-125b抑制物(中国广州锐博生物科技有限公司),苏木素、Masson试剂盒和免疫荧光试剂盒(美国Sigma公司),兔抗鼠心钠肽(ANP)单克隆抗体、兔抗鼠脑钠肽(BNP)单克隆抗体、兔抗鼠PI3K单克隆抗体、兔抗鼠Akt单克隆抗体和兔抗鼠磷酸化Akt (p-Akt)单克隆抗体(美国Abcam公司)。凝胶成像仪和电泳仪(美国Bio-Rad公司)。
1.2 实验动物分组、建模及处理75只大鼠随机分为假手术组、心肌梗死组和miR-125b抑制物组,每组25只。心肌梗死组和miR-125b抑制物组大鼠建立急性心肌梗死模型:将大鼠禁食12h后水合氯醛腹腔注射麻醉,气管插管、连接动物呼吸机,监测心电图;将大鼠备皮消毒后从左外侧经第3肋间隙切口进入胸腔,暴露大鼠心脏,结扎左冠状动脉前降支,心电图ST-T弓背向上抬、左室前壁颜色变白表示心肌梗死动物模型建立成功。假手术组大鼠前降支只穿线,不结扎。术后24h,miR-125b抑制物组大鼠经尾静脉注射miR-125b抑制物(80 mg·kg-1),假手术组和心肌梗死组大鼠经尾静脉注射等量生理盐水,每周1次,共4周。4周后假手术组大鼠死亡3只,模型组大鼠死亡5只,miR-125b抑制物组大鼠死亡4只。最终每组取20只大鼠进行各指标测量:每组取10只大鼠的心脏组织进行HE染色、Masson染色和免疫荧光染色;每组取剩余10只大鼠心脏组织用于左心室肥厚指数测定和Western blotting法测定。
1.3 左心室肥厚指数测定4周后电子天平称大鼠体质量,处死大鼠取心脏,剪去大血管和左右心耳,冲洗干净,电子天平秤称质量。剪去右心室和左右心房,将左心室及室间隔称质量,计算左心室肥厚指数,包括心脏质量/体质量(HW/BW)、(左心室+室间隔)质量/体质量[(LV+S)/BW]和(左心室+室间隔)质量/心脏质量[(LV+S)/HW]。
1.4 HE染色观察大鼠心肌组织形态表现处死大鼠,取心脏组织置于甲醛中固定,经脱水、石蜡包埋,切成厚度为4μm切片,常规进行HE染色,观察各组大鼠心肌组织形态表现。
1.5 Masson染色观察心肌组织纤维化情况将各组大鼠心肌组织烤片60 min,经脱蜡至水,苏木素染色10min,盐酸酒精分化10s,丽春红酸性品红染色5 min,苯胺蓝复染15 min,二甲苯透明,中性树胶封片,显微镜下拍片观察染色情况。蓝色为胶原纤维,红色为心肌细胞。采用ImagePro Plus 6.0软件计算大鼠心肌胶原容积积分,胶原容积积分=胶原面积/总面积×100%。
1.6 免疫荧光染色法观察大鼠心肌组织Ⅰ型胶原和Ⅲ型胶原蛋白表达水平将各组大鼠心肌组织切片加入丙酮固定,PBS液冲洗,山羊血清封闭,加入一抗(兔抗鼠Ⅰ型胶原蛋白单克隆抗体和兔抗鼠Ⅲ型胶原蛋白单克隆抗体)(1:1 000)过夜孵育,PBS冲洗,加入异硫氰酸荧光素标记的二抗(1:200)孵育1h,PBS冲洗,甘油封片,200倍光镜下观察免疫荧光染色情况。采用ImagePro Plus 6.0分析软件测定目标蛋白荧光强度即积分光密度(IOD)值,代表目的蛋白表达水平。
1.7 Western blotting法测定大鼠心肌梗死周边区组织中ANP、BNP、PI3K、Akt和p-Akt蛋白表达水平取3组大鼠左心室梗死和非梗死交界处心肌组织,采用全蛋白提取法提取心肌梗死周边区总蛋白,BCA法测定3组蛋白浓度,每组取50μg上样,SDS-PAGE电泳分离蛋白质,经转膜后加入一抗:兔抗鼠ANP单克隆抗体(1:600)、兔抗鼠BNP单克隆抗体(1:600)、兔抗鼠PI3K单克隆抗体(1:800)、兔抗鼠Akt单克隆抗体(1:700)和兔抗鼠p-Akt单克隆抗体(1:600)过夜孵育,加入荧光二抗(1:4000)孵育1h,以β-actin为内参照,采用Fusion生物凝胶图像分析系统扫描各条带吸光度(A)值。目的蛋白表达水平=目的蛋白条带A值/β-actin条带A值。
1.8 统计学分析采用SPSS20.0统计软件进行统计学分析。各组大鼠HW/BW、(LV+S)/BW和(LV+S)/HW值,心肌组织胶原容积积分,心肌组织中Ⅰ型胶原、Ⅲ型胶原、ANP、BNP、PI3K、Akt和p-Akt蛋白表达水平,均符合正态分布,以x±s表示,3组间样本均数比较采用单因素方差分析,组间两两比较采用SNK-q法。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠左心室肥厚指数与假手术组比较,心肌梗死组和miR-125b抑制物组大鼠HW/BW、(LV+S)/BW和(LV+S)/HW均明显升高(P < 0.05);与心肌梗死组比较,miR-125b抑制物组大鼠HW/BW、(LV+S)/BW和(LV+S)/HW明显降低(P < 0.05)。见表 1。
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | HW/BW(×10-4) | (LV+S)/BW(×10-4) | (LV+S)/HW(×10-2) | ||||||||||||||||||||||||||
| Sham operation | 23.51±0.71 | 18.79±0.65 | 77.14±1.03 | ||||||||||||||||||||||||||
| Myocardial infarction | 32.17±0.82* | 28.71±0.74* | 84.21±1.12* | ||||||||||||||||||||||||||
| MiR-125b inhibitor | 27.42±0.77*△ | 23.53±0.68*△ | 80.51±1.07*△ | ||||||||||||||||||||||||||
| F | 318.883 | 515.555 | 108.421 | ||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||||
| * P < 0.05 compared with sham operation group; △ P < 0.05 compared with myocardial infarction group. | |||||||||||||||||||||||||||||
假手术组大鼠心肌细胞排列整齐,细胞核明显;心肌梗死组大鼠心肌细胞排列紊乱,形态不规则,肿胀、增粗明显,细胞间隙可见大量网状分布胶原纤维增生,并有明显炎性细胞浸润;miR-125b抑制物组大鼠心肌细胞排列较为整齐。见图 1(插页六)。
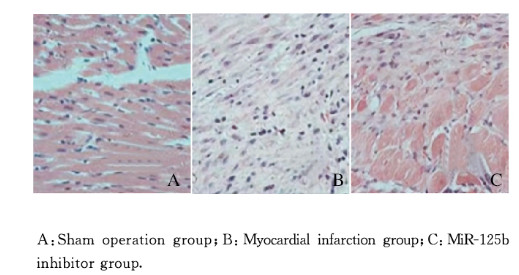
|
| 图 1 各组大鼠心肌组织形态表现(HE,×200) Fig. 1 Morphology of myocardium tissue of rats in various groups(HE, ×200) |
|
|
假手术组大鼠心肌组织呈均匀红色,无融合及条索状胶原纤维;心肌梗死组大鼠梗死区心肌组织可见大量胶原纤维,呈条索状分割心肌束;miR-125b抑制物组大鼠心肌组织胶原纤维明显减少。与假手术组(5.31%±1.24%)比较,心肌梗死组和miR-125b抑制物组大鼠心肌组织胶原容积积分(47.63%±1.18%和13.51%±0.98%)明显升高(P < 0.05);与心肌梗死组比较,miR-125b抑制物组大鼠心肌组织胶原容积积分明显降低(P < 0.05)。见图 2(插页六)。
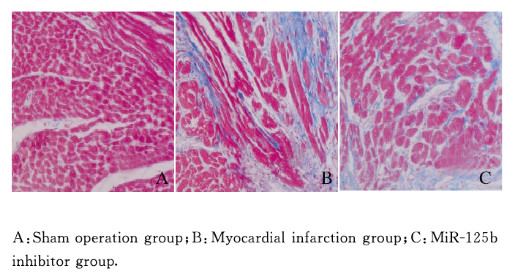
|
| 图 2 各组大鼠心肌组织Masson染色结果(×200) Fig. 2 Masson staining results of myocardium tissue of rats in various groups(×200) |
|
|
与假手术组比较,心肌梗死组和miR-125b抑制物组大鼠心肌组织Ⅰ型胶原和Ⅲ型胶原蛋白表达水平明显升高(P < 0.05);与心肌梗死组比较,miR-125b抑制物组大鼠心肌组织Ⅰ型胶原和Ⅲ型胶原蛋白表达水平明显降低(P < 0.05)。见表 2和图 3(插页六)。
| Group | Type Ⅰ collagen | Type Ⅲ collagen |
| Sham operation | 1.21±0.15 | 1.09±0.13 |
| Myocardial infarction | 2.43±0.22* | 2.25±0.17* |
| MiR-125b inhibitor | 1.74±0.19*△ | 1.66±0.16*△ |
| F | 104.925 | 141.359 |
| P | < 0.01 | < 0.01 |
| * P < 0.05 compared with sham operation group; △ P < 0.05 compared with myocardial infarction group. | ||

|
| 图 3 各组大鼠心肌组织中Ⅰ型胶原和Ⅲ型胶原免疫荧光染色结果(×200) Fig. 3 Immunofluorescence staining results of type Ⅰ collagen and type Ⅲ collagen in myocardium tissue of rats in various groups(×200) |
|
|
与假手术组比较,心肌梗死组和miR-125b抑制物组大鼠心肌梗死周边区组织中ANP和BNP蛋白表达水平明显升高(P < 0.05);与心肌梗死组比较,miR-125b抑制物组大鼠心肌梗死周边区组织中ANP和BNP蛋白表达水平明显降低(P < 0.05)。见表 3和图 4。
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | ANP protein | BNP protein | |||||||||||||||||||||||||||
| Sham operation | 0.11±0.03 | 0.09±0.02 | |||||||||||||||||||||||||||
| Myocardial infarction | 0.37±0.08* | 0.53±0.08* | |||||||||||||||||||||||||||
| MiR-125b inhibitor | 0.20±0.07*△ | 0.22±0.06*△ | |||||||||||||||||||||||||||
| F | 42.869 | 147.404 | |||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | |||||||||||||||||||||||||||
| * P < 0.05 compared with sham operation group; △ P < 0.05 compared with myocardial infarction group. | |||||||||||||||||||||||||||||

|
| Lane 1:Sham operation group; Lane 2:Myocardial infarction group; Lane 3:MiR-125b inhibitor group. 图 4 各组大鼠心肌梗死周边区组织中ANP和BNP蛋白表达电泳图 Fig. 4 Electrophoregram of expressions of ANP and BNP proteins in peripheral region tissue of myocardial infarction of rats in various groups |
|
|
各组大鼠心肌梗死周边区组织中Akt蛋白表达水平比较差异无统计学意义(P>0.05),PI3K和p-Akt蛋白表达水平比较差异有统计学意义(P < 0.01)。与假手术组比较,心肌梗死组和miR-125b抑制物组大鼠心肌梗死周边区组织中PI3K和p-Akt蛋白表达水平明显降低(P < 0.05);与心肌梗死组比较,miR-125b抑制物组大鼠心肌梗死周边区组织中PI3K和p-Akt蛋白表达水平明显升高(P < 0.05)。见表 4和图 5。
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | PI3K protein | Akt protein | p-Akt protein | ||||||||||||||||||||||||||
| Sham operation | 0.41±0.09 | 0.37±0.11 | 0.67±0.12 | ||||||||||||||||||||||||||
| Myocardial infarction | 0.12±0.05* | 0.39±0.10 | 0.23±0.07* | ||||||||||||||||||||||||||
| MiR-125b inhibitor | 0.26±0.10*△ | 0.43±0.09 | 0.36±0.11*△ | ||||||||||||||||||||||||||
| F | 30.631 | 0.927 | 48.822 | ||||||||||||||||||||||||||
| P | < 0.01 | 0.408 | < 0.01 | ||||||||||||||||||||||||||
| * P < 0.05 compared with sham operation group; △ P < 0.05 compared with myocardial infarction group. | |||||||||||||||||||||||||||||
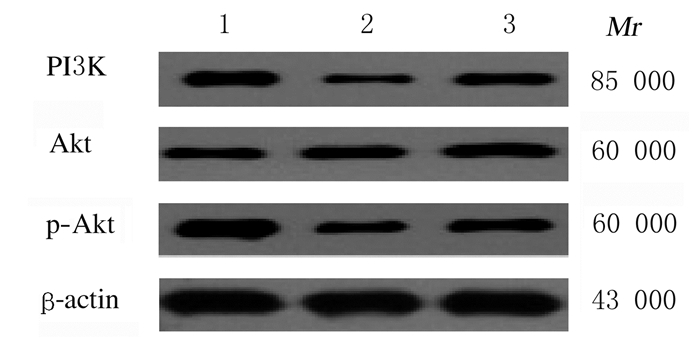
|
| Lane 1:Sham operation group; Lane 2:Myocardial infarction group; Lane 3:MiR-125b inhibitor group. 图 5 各组大鼠心肌梗死周边区组织中PI3K、Akt和p-Akt蛋白表达电泳图 Fig. 5 Electrophoregram of expessions of PI3K, Akt and p-Akt proteins in peripheral region tissue of myocardial infarction of rats in various groups |
|
|
心肌梗死可引起心肌成纤维细胞、心肌细胞和细胞外基质代偿性活化,分泌大量胶原蛋白,损伤心肌组织功能,造成心肌纤维化和心室重构等病理过程,最终导致心力衰竭的发生[7-8]。心脏非梗死区心肌纤维化是心室重构进展的关键环节[9],因此研究心肌细胞纤维化的调控机制对于防治心力衰竭具有重要作用。
miRNA为内源性RNA,相对分子质量小,可结合靶mRNA的编码区、3′端非编码区和5′端非编码区,在转录后对相关基因的表达进行调控,降解或抑制靶mRNA翻译,参与细胞增殖、分化和凋亡等多种病理生理进程[10]。近年来研究[11-12]显示:miRNA在心血管疾病及心脏病理生理过程中发挥重要作用,在心力衰竭和心肌肥厚等心血管疾病中均存在miRNA表达异常,在心室重构中也存在miRNA异常表达。miR-125b在心肌纤维化中具有重要作用,miR-125b模拟物转染可上调成人心脏成纤维细胞中Ⅰ型胶原、Ⅲ型胶原和α-平滑肌肌动蛋白水平,促进成人心脏成纤维细胞增殖、迁移和凋亡,通过转化生长因子β1(TGF-β1)/Smad通路参与心肌纤维化进程[13]。miR-125b可介导预防心肌梗塞中的细胞死亡促进心脏修复[14]。本研究结果显示:心肌梗死大鼠左心室肥厚指数和胶原容积积分升高,Ⅰ型胶原、Ⅲ型胶原、ANP和BNP蛋白水平表达水平明显升高。ANP和BNP为重要的心脏内分泌激素,梗死交界区ANP和BNP水平可反映心肌梗死局部张力,是心肌细胞病理性肥大的比较可靠的指标,在判定心室重构中具有重要价值[15-16]。本研究结果表明:心肌梗死模型大鼠心肌肥厚,存在心肌纤维化和心室重构,给予miR-125b抑制物处理后,心肌梗死模型大鼠左心室肥厚指数和胶原容积积分降低,Ⅰ型胶原、Ⅲ型胶原、ANP和BNP蛋白水平降低,表明抑制miR-125b可抑制心肌梗死模型大鼠心肌肥厚,改善心肌纤维化和心室重构。
miR-125b参与心肌纤维化和心室重构过程,但其作用机制尚不清楚。miR-125b可通过多种信号通路参与疾病的发生发展过程,过表达miR-125b可通过PI3K/Akt信号通路抑制子宫内膜癌HEC-1B细胞周期和细胞增殖,抑制细胞凋亡[17]。PI3K/Akt信号通路参与心肌纤维化进程,如调节第10号染色体缺失性磷酸酶-张力蛋白同源性基因(PTEN)-PI3K / AKT信号通路可调节心肌梗死后的心肌纤维化[18];PI3K/Akt通路介导TRPV1抑制离体大鼠心脏缺血再灌注后的细胞凋亡[19];PI3K/AKT信号通路在甲状腺素诱导的大鼠心肌细胞凋亡中具有重要作用[20]。本研究结果显示:心肌梗死后心室重构大鼠心肌梗死周边区组织中PI3K和p-Akt蛋白表达水平明显降低,给予miR-125b抑制物处理后心肌梗死大鼠心肌梗死周边区组织中PI3K和p-Akt蛋白表达水平升高,表明心肌梗死后心室重构大鼠心肌梗死周边存在PI3K/Akt信号通路抑制,抑制miR-125b可通过激活PI3K/Akt信号通路发挥抑制心肌纤维化和心室重构的作用。
综上所述,抑制miR-125b的表达可抑制心肌梗死后心室重构进程,其机制可能与激活PI3K/Akt信号通路有关。
| [1] |
GARBER L, MCANDREW T C, CHUNG E S, et al. Predictors of leftventricular remodeling after myocardial infarction in patients with a patent infarct related coronary artery after percutaneous coronary intervention (from the post-myocardial infarction remodeling prevention therapy[J]. Am J Cardiol, 2018, 121(11): 1293-1298. DOI:10.1016/j.amjcard.2018.02.007 |
| [2] |
MCCARROLL C S, HE W H, FOOTE K, et al. Runx1 deficiency protects against adverse cardiac remodeling after myocardial infarction[J]. Circulation, 2018, 137(1): 57-70. DOI:10.1161/CIRCULATIONAHA.117.028911 |
| [3] |
BIE Z D, SUN L Y, GENG C L, et al. MiR-125bregulates SFRP5 expression to promote growth and activation of cardiac fibroblasts[J]. Cell Biol Int, 2016, 40(11): 1224-1234. DOI:10.1002/cbin.10677 |
| [4] |
别自东, 王东, 何琳, 等. 微小RNA-125b作用于SFRP5调节急性心肌梗死后心室重构的实验研究[J]. 中西医结合心脑血管病杂志, 2017, 15(11): 1315-1319. DOI:10.3969/j.issn.1672-1349.2017.11.008 |
| [5] |
RIQUELME I, TAPIA O, LEAL P, et al. miR-101-2, miR-125b-2 and miR-451a act as potential tumor suppressors in gastric cancer through regulation of the PI3K/AKT/mTOR pathway[J]. Cell Oncol (Dordr), 2016, 39(1): 23-33. |
| [6] |
LI J W, WANG X Y, ZHANG X, et al. Epicatechin protects against myocardial ischemia induced cardiac injury via activation of the PTEN/PI3K/AKT pathway[J]. Mol Med Rep, 2018, 17(6): 8300-8308. |
| [7] |
ZHANG L, MANDRY D, CHEN B L, et al. Impact of microvascular obstruction on left ventricular local remodeling after reperfused myocardial infarction[J]. J Magn Reson Imaging, 2018, 47(2): 499-510. DOI:10.1002/jmri.25780 |
| [8] |
CASTELVECCHIO S, MORONI F, MENICANTI L. The matter of reverse ventricular remodeling after acutemyocardial infarction between fiction and reality[J]. J Cardiovasc Med (Hagerstown), 2018, 19(8): 397-398. DOI:10.2459/JCM.0000000000000658 |
| [9] |
CHOE J C, CHA K S, YUN E Y, et al. Reverse left ventricular remodelling in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention:incidence, predictors, and impact on outcome[J]. Heart Lung Circ, 2018, 27(2): 154-164. DOI:10.1016/j.hlc.2017.02.020 |
| [10] |
LUO S, CHEN Y, HE R, et al. Rescuing infusion of miRNA-1 prevents cardiac remodeling in a heart-selective miRNA deficient mouse[J]. Biochem Biophys Res Commun, 2018, 495(1): 607-613. DOI:10.1016/j.bbrc.2017.11.029 |
| [11] |
MOHSENI Z, SPAANDERMAN M E A, OBEN J, et al. Cardiac remodeling and pre-eclampsi:An overview of microRNA expression patterns[J]. Ultrasound Obstet Gynecol, 2018, 52(3): 310-317. DOI:10.1002/uog.17516 |
| [12] |
VERJANS R, PETERS T, BEAUMONT F J, et al. MicroRNA-221/222 family counteracts myocardial fibrosis in pressure overload-induced heart failure[J]. Hypertension, 2018, 71(2): 280-288. DOI:10.1161/HYPERTENSIONAHA.117.10094 |
| [13] |
沈志方, 许学升, 孙继兰. MiR-125b对心肌梗死后成纤维细胞的调控机制研究[J]. 中国循证心血管医学杂志, 2018, 10(7): 853-856, 860. DOI:10.3969/j.issn.1674-4055.2018.07.21 |
| [14] |
ZHU L P, TIAN T, WANG J Y, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction[J]. Theranostics, 2018, 8(22): 6163-6177. DOI:10.7150/thno.28021 |
| [15] |
TURKIEH A, FERTIN M, BOUVET M, et al. Expression and implication of clusterin in left ventricular remodeling after myocardial infarction[J]. Circ Heart Fail, 2018, 11(6): e004838. |
| [16] |
PERSOON S, PAULUS M, HIRT S, et al. Cardiac unloading by LVAD support differentially influences components of the cGMP-PKG signaling pathway in ischemic and dilated cardiomyopathy[J]. Heart Vessels, 2018, 33(8): 948-957. DOI:10.1007/s00380-018-1149-x |
| [17] |
常军, 刘玲芳, 郑殊娟, 等. 过表达miR-125b对子宫内膜癌HEC-1B细胞周期、增殖和凋亡的影响及其可能的机制[J]. 中国肿瘤生物治疗杂志, 2014, 21(3): 303-308. |
| [18] |
ZHANG S, CUI R. The targeted regulation of miR-26a on PTEN-PI3K/AKT signaling pathway in myocardial fibrosis after myocardial infarction[J]. Eur Rev Med Pharmacol Sci, 2018, 22(2): 523-531. |
| [19] |
姜小雪, 刘关羽, 雷寒, 等. PI3K/Akt通路介导TRPV1抑制离体大鼠心脏缺血再灌注后的细胞凋亡[J]. 基础医学与临床, 2016, 36(9): 1187-1192. |
| [20] |
LIU M J, LI Z N, LIANG B, et al. Hydrogen sulfide ameliorates rat myocardial fibrosis induced by thyroxine through PI3K/AKT signaling pathway[J]. Endocr J, 2018, 65(7): 769-781. DOI:10.1507/endocrj.EJ17-0445 |
 2020, Vol. 46
2020, Vol. 46


