扩展功能
文章信息
- 李雪洋, 尹硕, 谢金芳, 李晶, 胡雪, 耿文韬, 张颖丽
- LI Xueyang, YIN Shuo, XIE Jinfang, LI Jing, HU Xue, GENG Wentao, ZHANG Yingli
- 促红细胞生成素对大鼠即刻再植牙牙髓血运重建的促进作用
- Promotion effect of erythropoietin on immediate revascularization of replanted teeth in rats
- 吉林大学学报(医学版), 2020, 46(01): 56-60
- Journal of Jilin University (Medicine Edition), 2020, 46(01): 56-60
- 10.13481/j.1671-587x.20200110
-
文章历史
- 收稿日期: 2019-07-07
2. 吉林省长春市口腔医院修复科, 吉林 长春 130022
2. Department of Prosthodontics, Changchun Stomatology Hospital, Jilin Province, Changchun 130022, China
牙外伤作为临床上十分普遍的牙急性损伤,多发生于年轻恒牙[1], 而牙外伤中以牙脱位较为常见,此时青少年正处于生长发育期,根尖孔尚未完全闭合。牙脱位会带来美学、功能和心理上的负担,鉴于生物学和心理学上的支持[2],国际牙外伤协会(IADT)指南建议即刻再植是治疗牙脱位的最佳方法,同时该指南指出为了获得牙髓血运重建和牙根的持续发育,对根尖孔开放的全脱位牙来说,除非有临床或影像学证据显示牙髓坏死,否则应避免进行根管治疗[3]。MELO等[4]研究表明:在理想的情况下,牙髓的血运重建和牙根的继续发育是可能发生的,因此再植牙的牙髓治疗并不适用于牙根尚未发育完全的牙齿。随着研究的深入,人们逐渐认识到牙髓细胞具有再生潜能,细胞迁移和血管新生在牙髓修复再生中均具有重要的作用[5-6]。因此,如何诱导牙髓组织内血管新生以发挥牙髓的防御修复潜能,是牙髓修复再生和临床活髓保存研究的重点。研究[7-10]显示:促红细胞生成素(erythropoietin,EPO)可通过募集基质细胞至受损区域促进组织的再生修复,体内外实验也已证实EPO在脑缺血再灌注的保护、烧伤皮肤的愈合和股骨头坏死修复中均发挥了促进血管新生的作用。目前对于再植牙的研究则大多集中于储存介质和牙周组织的愈合[11],而EPO在再植牙牙髓修复方面的研究较少。因此本研究旨在探讨EPO对再植牙牙髓血运重建的影响,为研究牙髓损伤的修复再生提供理论依据。
1 材料与方法 1.1 实验动物、主要试剂及仪器80只3周龄雄性Wistar大鼠购自吉林大学实验动物中心,动物许可证号:SCXF(吉)2015-0001,标准饲粮、随机加水喂养,使其适应环境1周,术前体质量约100 g。EPO(沈阳三生制药有限责任公司),庆大霉素(上海现代哈森药业有限公司),生理盐水(辽宁民康药业有限公司),血管内皮细胞生长因子(vascular endothelial cell growth factor,VEGF)抗体和SABC免疫组织化学染色试剂盒(武汉博士生物工程有限公司),DAB显色试剂盒(北京中山金桥生物技术有限公司)。双目光学显微镜(吉林大学口腔医院病理教研室提供,配有日本QLYMPUS照相机)。ImageProPlus6.0(For windows)专业图像分析软件包,SPSS24.0统计软件。
1.2 实验动物分组和给药80只4周龄雄性Wistar大鼠随机分为未拔牙组、阴性对照(生理盐水)组、阳性药对照(庆大霉素)组和EPO组,再根据观察时间随机分为3、7、14、21和28 d组。除外未拔牙组,其他3组大鼠固定后用30 g ·L-1水合氯醛(30 mL·kg-1体质量)腹腔注射麻醉,显效后用自制拔牙钳完整拔出上颌第一磨牙,将拔出的牙齿置于无菌一次性器械盘中,分别在生理盐水、庆大霉素和EPO溶液中浸泡4 min,再轻柔地植回牙槽窝内,实现牙齿在5 min内再植。牙再植术后严密观察大鼠复苏情况,自由饮水和摄食,每100 g饲料中混入阿莫西林0.5g,疗程为1周。
1.3 标本采集和处理分别在再植术后3、7、14、21和28 d取各组大鼠上颌第一磨牙及其周围组织,用4%多聚甲醛溶液4℃下固定24~48h,于10%EDTA溶液中脱钙12周,然后将标本放入梯度乙醇中脱水,浸蜡,包埋,通过牙齿颊舌面沿牙齿长轴过根尖孔做厚度为3μm的连续切片。
1.4 免疫组织化学染色和HE染色将石蜡切片放入烤箱中2 h,常规脱蜡水化,PBS冲洗后用3%双氧水灭活10 min,PBS洗3次;再放入EDTA抗原修复液中进行高压修复2 min,待冷却至室温时,PBS洗3次;随后滴加5%BSA封闭液封闭30 min,滴加VEGF抗体过夜;隔天PBS洗3次后滴加二抗30 min,PBS冲洗后滴加SABC 30 min,PBS冲洗后滴加DAB显示剂,显微镜下观察反应,终止后复染、分色、返蓝和透明,中性树脂封片,400倍显微镜下观察拍照,观察VEGF阳性表达情况,用ImageProPlus6.0专业图像分析软件包测定上述切片中VEGF平均吸光度(AOD)值,代表VEGF蛋白表达水平。经HE染色,中性树脂封片,200倍显微镜下观察再植牙的牙根发育情况。
1.5 统计学分析采用SPSS24.0统计软件进行统计学分析。大鼠再植牙牙体组织中VEGF蛋白表达水平以x±s表示,组间比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 免疫组织化学染色检测各组大鼠牙体组织中VEGF蛋白表达各组大鼠再植牙牙体组织中,成牙本质细胞、前期牙本质、血管内皮细胞和牙髓细胞中VEGF蛋白呈阳性表达;与固有髓核比较,各组大鼠牙体组织中VEGF蛋白在成牙本质细胞层阳性表达出现的时间较早且较强。与未拔牙组比较,其他3组大鼠再植牙牙体组织在3、7和14 d时VEGF蛋白呈强阳性表达,随着时间的推移,VEGF蛋白阳性表达强度逐渐减弱。与未拔牙组和生理盐水组比较,庆大霉素组和EPO组大鼠再植牙牙体组织中VEGF蛋白的表达均较强。见图 1(插页四)。
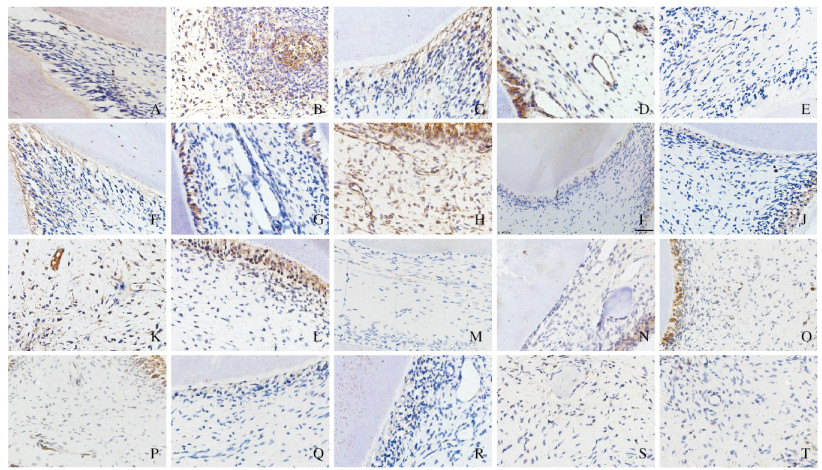
|
| 图 1 术后不同时间各组大鼠再植牙牙体组织中VEGF蛋白表达情况(免疫组织化学,×400) Fig. 1 Expressions of VEGF protein in tooth tissue of replanted teeth of rats in various groups at different time after operation(Immunohistochemistry, ×400) |
|
|
未拔牙组、生理盐水组、庆大霉素组和EPO组大鼠再植牙牙体组织的AOD值从高到低依次为:EPO组>庆大霉素组>生理盐水组>未拔牙组。与未拔牙组比较,3、7、14和21 d时生理盐水组、庆大霉素组和EPO组大鼠再植牙牙体组织中VEGF蛋白表达水平明显升高(P < 0.05),而28 d时各组大鼠再植牙牙体组织中VEGF蛋白表达水平差异无统计学意义(P > 0.05);与生理盐水组比较,3、7、14和21 d时庆大霉素组和EPO组大鼠再植牙牙体组织VEGF蛋白表达水平明显升高(P < 0.05),而在28d时VEGF蛋白表达水平差异无统计学意义(P > 0.05);与庆大霉素组比较,术后各时间点EPO组大鼠再植牙牙体组织中VEGF蛋白表达水平差异均无统计学意义(P > 0.05)。见表 1。
| (n=4, x±s) | |||||
| Group | Expression level of VEGF protein | ||||
| (t/d) 3 | 7 | 14 | 21 | 28 | |
| Non-tooth extraction | 0.123±0.012 | 0.112±0.014 | 0.116±0.016 | 0.091±0.015 | 0.095±0.024 |
| Normal saline | 0.171±0.032* | 0.171±0.024* | 0.156±0.019* | 0.108±0.014* | 0.093±0.021 |
| Gentamycin | 0.237±0.019*△ | 0.246±0.038*△ | 0.233±0.041*△ | 0.153±0.051*△ | 0.104±0.020 |
| EPO | 0.260±0.048*△ | 0.263±0.085*△ | 0.239±0.029*△ | 0.158±0.027*△ | 0.091±0.010 |
| * P < 0.05 vs non-tooth extraction group; △ P < 0.05 vs normal saline group. | |||||
再植术后3d,未拔牙组大鼠再植牙牙根持续发育,血运丰富;生理盐水组大鼠再植牙可见炎症浸润灶;庆大霉素组大鼠再植牙纤维结缔组织长入;与未拔牙组和生理盐水组比较,EPO组大鼠再植牙血管内皮细胞和血管腔数目明显增多。再植术后7d,未拔牙组大鼠再植牙牙本质增厚;生理盐水组大鼠再植牙可见疏松结缔组织长入;庆大霉素组大鼠再植牙血管腔增多;与未拔牙组比较,EPO组大鼠再植牙可见钙化组织和修复性牙本质;与生理盐水组比较,庆大霉素组和EPO组大鼠再植牙的根尖孔均缩小。再植术后14 d,生理盐水组大鼠再植牙血管充血扩张;庆大霉素组大鼠再植牙开始出现钙化;与未拔牙组比较,EPO组大鼠再植牙髓腔内出现类骨样组织,牙本质小管排列紊乱。再植术后21 d,未拔牙组和EPO组大鼠再植牙根管壁持续增厚;生理盐水组大鼠再植牙出现牙髓坏死;庆大霉素组大鼠再植牙可见牙骨质和修复性牙本质沉积,根尖孔缩小。再植术后28 d,未拔牙组和EPO组大鼠再植牙牙根逐渐发育成熟;生理盐水组大鼠再植牙牙骨质处可见骨吸收陷窝和破骨细胞,出现牙根吸收;庆大霉素组大鼠再植牙髓腔内出现类骨样组织。见图 2(插页四)。
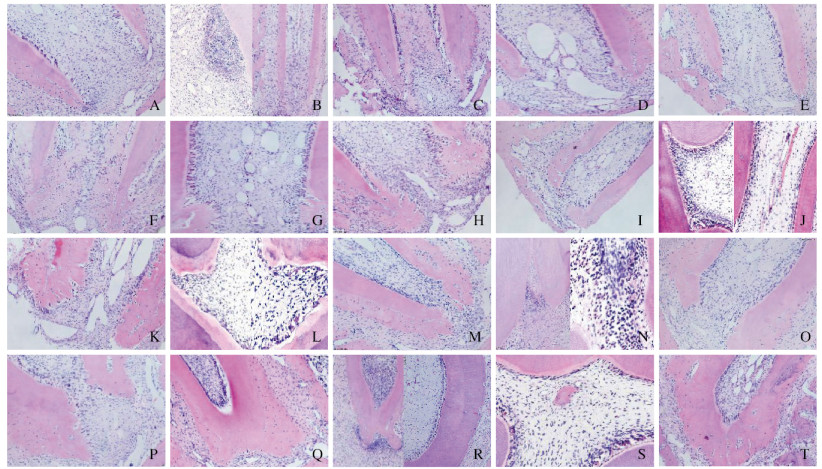
|
| A-D:3 d; E-H:7 d; I-L:14 d; M-P:21 d; Q-T:28 d; A, E, I, M, Q:Non-tooth extraction group; B, F, J, N, R:Saline group; C, G, K, O, S:Gentamycin group; D, H, L, P, T:EPO group. 图 2 术后不同时间各组大鼠再植牙牙体组织形态表现(HE,×200) Fig. 2 Morphology of tooth tissue of replanted teeth of rats in various groups at different time after operation(HE, ×200) |
|
|
对于牙根未发育成熟的脱位牙,实现即刻再植或再植前储存在合适的介质中,牙髓血运重建是有可能发生的[3]。研究[12-13]表明:大鼠的上颌第一磨牙在15 d时牙根开始发育,25~30 d时牙根形成1/2~2/3,大鼠的切牙末端存在被称为“apical bud”的特殊上皮结构,使切牙得以终生不断萌出。此外,有文献[14-15]指出:牙髓血运重建通常在再植后30 d左右建立,且牙周膜的修复在28 d完成。因此本实验选择4周龄大鼠的第一磨牙进行了为期28 d的观察,排除了个体自身发育的影响。
近年来研究者[16]发现了VEGF在人牙髓成纤维细胞中的表达。本研究结果显示:无论是对照组还是实验组,大鼠牙体组织中VEGF均呈阳性表达。还有研究[17]显示:牙本质基质中含有VEGF,其在损伤后从牙本质基质中释放,从而起到修复牙髓-牙本质复合体的作用。同时,成牙本质细胞的体外培养研究[18]表明:成牙本质细胞可以上调VEGF的表达。在本研究中,与固有髓核比较,成牙本质细胞层阳性表达出现的时间较早且阳性表达较强。
随着对VEGF研究的深入,有学者[19]发现:EPO通过蛋白质酪氨酸激酶2/信号传导转录激活因子3(JAK2/ STAT3)信号通路上调VEGF的表达。本研究结果显示:在再植术后3、7和14 d时EPO组和庆大霉素组大鼠再植牙牙体组织中VEGF均呈强阳性表达,可能与该时间内成牙本质细胞变性、炎症、牙髓间充质干细胞迁移和分化、牙髓血运重建和修复性牙本质形成活跃有关[5, 15, 20-21];EPO组大鼠再植牙牙体组织中VEGF表达水平略强于庆大霉素组,但差异无统计学意义,推测可能是因为在本实验中实现了即刻再植,与现实条件比较,操作均在相对无菌的条件下进行,细菌污染程度相对较轻。体外研究[22]显示:牙髓炎时,牙髓组织中EPO及其受体呈强阳性表达,表明EPO在炎症牙髓中发挥着一定的作用。并且EPO除了抗炎作用外,还具有促进血管再生、神经保护和促进成骨[7-10]的作用。最近的研究[23]表明:EPO通过促进Runt相关转录因子2(Runx2)、碱性磷酸酶(ALP)和骨钙素的表达以及促分裂素原活化蛋白激酶(mitogen-activated protein kinases, MAPK)途径上调人牙周膜间充质干细胞和牙周炎间充质干细胞的成骨能力。但本研究选择的是VEGF作为观察指标,而相关的研究[16]表明:VEGF的表达与牙髓炎中血管化的增加相一致,因此并不能说明EPO在增加再植牙成功率方面优于庆大霉素,还需要长期的观察和增加样本量以及检测细胞因子进行验证,但根据庆大霉素组和EPO组大鼠再植牙牙体组织中VEGF蛋白表达水平均强于生理盐水组和未拔牙组的结果推测,将VEGF和抗生素结合的微球作用于再植牙,可能会提高再植牙的远期成活率,这将成为本课题组未来的研究方向。
综上所述,本研究结果表明:EPO对再植牙的牙髓血运重建起到了一定的促进作用。
| [1] |
姚亚男, 汪国华, 姚华. 牙髓血管再生术临床应用的研究进展[J]. 吉林大学学报(医学版), 2014, 40(6): 1330-1334. |
| [2] |
LOO T J, GURUNATHAN D, SOMASUNDARAM S. Knowledge and attitude of parents with regard to avulsed permanent tooth of their children and their emergency management:Chennai[J]. J Indian Soc Pedod Prev Dent, 2014, 32(2): 97-107. DOI:10.4103/0970-4388.130781 |
| [3] |
DIANGELIS A J, ANDREASEN J O, EBELESEDER K A, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries:1. Fractures and luxations of permanent teeth[J]. Dent Traumatol, 2012, 28(1): 2-12. DOI:10.1111/j.1600-9657.2011.01103.x |
| [4] |
MELO M E, SILVA C A, DE SOUZA GOMES W D, et al. Immediate tooth replantation in rats:effect of systemic antibiotic therapy with amoxicillin and tetracycline[J]. Clin Oral Investig, 2016, 20(3): 523-532. DOI:10.1007/s00784-015-1534-0 |
| [5] |
AL-SHARABI N, XUE Y, UDEA M, et al. Influence of bone marrow stromal cell secreted molecules on pulpal and periodontal healing in replanted immature rat molars[J]. Dent Traumatol, 2016, 32(3): 231-239. DOI:10.1111/edt.12246 |
| [6] |
FURFARO F, ANG E S, LAREU R R, et al. A histological and micro-CT investigation in to the effect of NGF and EGF on the periodontal, alveolar bone, root and pulpal healing of replanted molars in a rat model-a pilot study[J]. Prog Orthod, 2014, 15: 2. DOI:10.1186/2196-1042-15-2 |
| [7] |
CHEN T L, CHIANG Y W, LIN G L, et al. Different effects of granulocyte colony-stimulating factor and erythropoietin on erythropoiesis[J]. Stem Cell Res Ther, 2018, 9(1): 119-127. DOI:10.1186/s13287-018-0877-2 |
| [8] |
HABIBA P, STAMMA A, ZEYEN T, et al. EPO regulates neuroprotective transmembrane BAX inhibitor-1 motifcontaining (TMBIM) family members GRINA and FAIM2 after cerebral ischemia-reperfusion injury[J]. Exp Neurol, 2019, 320: 112978. DOI:10.1016/j.expneurol.2019.112978 |
| [9] |
HAMED S, ULLMANN Y, EGOZI D, et al. Topical erythropoietin treatment accelerates the healing of cutaneous burn wounds in diabetic pigs through an aquaporin-3-dependent mechanism[J]. Diabetes, 2017, 66(8): 2254-2265. DOI:10.2337/db16-1205 |
| [10] |
YAN Y Q, PANG Q J, XU R J. Effects of erythropoietin for precaution of steroid-induced femoral head necrosis in rats[J]. BMC Musculoskelet Disord, 2018, 19(1): 282. DOI:10.1186/s12891-018-2208-2 |
| [11] |
MOURA C C, SOARES P B, DE PAULA REIS M V, et al. Potential of coconut water and soy milk for use as storage media to preserve the viability of periodontal ligament cells:An in vitro study[J]. Dent Traumatol, 2014, 30(1): 22-26. DOI:10.1111/edt.12042 |
| [12] |
LOSSO E M, JOSE NICOLAU J. Lactate dehydrogenase isoenzymes in dental pulp of rats according to stage of root development[J]. Braz Dent J, 2003, 14(1): 5-11. DOI:10.1590/S0103-64402003000100001 |
| [13] |
白玉娣.大鼠切牙Apical bud和磨牙Hertwig's上皮根鞘细胞生物学性质研究[D].西安: 第四军医大学, 2008. http://cdmd.cnki.com.cn/Article/CDMD-90026-2008197055.htm
|
| [14] |
STROBL H, GOJER G, NORER B, et al. Assessing revascularization of avulsed permanent maxillary incisors by laser Doppler flowmetry[J]. J Am Dent Assoc, 2003, 134(12): 1597-1603. DOI:10.14219/jada.archive.2003.0105 |
| [15] |
PANZARINI S R, OKAMOTO R, POI W R, et al. Histological and immunohistochemical analyses of the chronology of healing process after immediate tooth replantation in incisor rat teeth[J]. Dent Traumatol, 2013, 29(1): 15-22. DOI:10.1111/j.1600-9657.2012.01127.x |
| [16] |
NAKANISHI T, MUKAI K, HOSOKAWA Y, et al. Catechins inhibit vascular endothelial growth factor production and cyclooxygenase-2 expression in human dental pulp cells[J]. Int Endod J, 2015, 48(3): 277-282. DOI:10.1111/iej.12312 |
| [17] |
ROBERTS-CLARK D J, SMITH A J. Angiogenic growth factors in human dentine matrix[J]. Arch Oral Biol, 2000, 45(11): 1013-1016. DOI:10.1016/S0003-9969(00)00075-3 |
| [18] |
BOTERO T M, SHELBURNE C E, HOLLAND G R, et al. TLR4 mediates LPS-induced VEGF expression in odontoblasts[J]. Endod, 2006, 32(10): 951-955. DOI:10.1016/j.joen.2006.03.018 |
| [19] |
WESTENBRINK B D, RUIFROK W P, VOORS A A, et al. Vascular endothelial growth factor is crucial for erythropoietin-induced improvement of cardiac function in heart failure[J]. Cardiovasc Res, 2010, 87(1): 30-39. |
| [20] |
SAITO K, OHSHIMA H. Differentiation capacity and maintenance of dental pulp stem/progenitor cells in the process of pulpal healing following tooth injuries[J]. J Oral Biosci, 2017, 59(2): 63-70. DOI:10.1016/j.job.2017.03.001 |
| [21] |
SHIMIZU A, NAKAKURA-OHSHIMA K, NODA T, et al. Responses of immunocompetent cells in the dental pulp to replantation during the regeneration process in rat molars[J]. Cell Tissue Res, 2000, 302(2): 221-233. |
| [22] |
GONG Q M, JIANG H W, WEI X, et al. Expression of erythropoietin and erythropoietin receptor in human dental pulp[J]. J Endod, 2010, 36(12): 1972-1977. DOI:10.1016/j.joen.2010.08.041 |
| [23] |
WANG L Y, WU F, SONG Y, et al. Erythropoietin induces the osteogenesis of periodontal mesenchymal stem cells from healthy and periodontitis sources via activation of the p38 MAPK pathway[J]. Int J Mol Med, 2018, 41(2): 829-835. |
 2020, Vol. 46
2020, Vol. 46


