扩展功能
文章信息
- 杜兴旭, 乔子敬, 杨硕, 许智莹, 杨波, 李贺, 陈建光, 王春梅
- DU Xingxu, QIAO Zijing, YANG Shuo, XU Zhiying, YANG Bo, LI He, CHEN Jianguang, WANG Chunmei
- 五味子多糖对2型糖尿病大鼠血清中炎症因子的影响及其作用机制
- Effect of Schisandra chinensis polysaccharide on serum inflammatory factors in T2DM rats and its mechanism
- 吉林大学学报(医学版), 2020, 46(01): 50-55
- Journal of Jilin University (Medicine Edition), 2020, 46(01): 50-55
- 10.13481/j.1671-587x.20200109
-
文章历史
- 收稿日期: 2019-03-05
2. 北华大学药学院药理学教研室, 吉林 吉林 132013
2. Department of Pharmacology, College of Pharmacy, Beihua University, Jilin 132013, China
2型糖尿病(type 2 diabetic mellitus, T2DM)的主要病理特征是胰岛素抵抗(insulin resistance, IR)和胰岛β细胞功能衰竭。随着对T2DM和IR研究的不断深入,发现炎症反应已成为IR、胰岛β细胞功能减退、代谢综合征和心血管并发症的“共同土壤”[1]。2011年,DONATH等[2]研究表明:T2DM是一种炎症性疾病。大量研究[3-5]表明:炎症因子如白细胞介素类、肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)、C反应蛋白(C-reactive protein, CRP)和核因子-κB (nuclear factor-κB, NF-κB)等可以通过血液和(或)旁分泌的作用影响胰岛素(insulin, INS)的敏感性和胰岛β细胞功能,因此抑制上述炎症因子能够起到改善IR、保护胰岛β细胞和降低血糖的作用[6-7]。
五味子[Schisandra chinensis(Turcz.) Baill.]是著名的长白山道地药材,具有酸、苦、甘、辛和咸5种药性,且以酸味为主,能补益五脏,是治疗糖尿病之上品[8]。近年来研究[9-10]显示:五味子治疗糖尿病效果明显,不仅可以降低糖尿病大鼠血糖和血脂水平,还能提高靶组织对INS的敏感性,延缓胰岛β细胞凋亡,改善β细胞的功能。然而,五味子发挥抗糖尿病作用的具体成分和机制尚不清楚。多糖是五味子的主要活性成分之一,具有抗氧化、调节血脂和保护肝脏等功效[11-13]。本课题组前期研究[14]表明:五味子多糖(Schisandra chinensis polysaccharide, SCP)对链脲佐菌素(streptozotocin, STZ)和四氧嘧啶诱导的小鼠糖尿病均具有明显的降血糖作用,且能降低血清中TNF-α和白细胞介素1β(interleukin-1β,IL-1β)水平。为了进一步探讨SCP对T2DM的治疗作用及其与抗炎症反应的关系,本研究采用高脂饮食联合小剂量STZ建立T2DM大鼠模型,观察SCP对T2DM的治疗作用,并探讨SCP的降糖作用是否与其抑制炎症反应有关,为五味子的进一步开发和利用奠定理论基础。
1 材料与方法 1.1 实验动物、药物、主要试剂和仪器雄性Wistar大鼠70只,体质量为180~230 g,由长春亿斯实验动物技术有限责任公司提供,动物许可证号:SCXK(吉)2016-0003。大鼠采取分笼饲养,自由摄食饮水,室温21℃~24℃,湿度40%~60%,适应环境5 d后用于实验。实验大鼠的普通饲料和高脂饲料均购于吉林省长春市亿斯实验动物技术有限责任公司,高脂饲料含碳水化合物53.3%、酪蛋白20.0%、猪油10.0%、蔗糖5.0%、胆固醇2.0%和其他9.7%。
SCP由东北师范大学生命科学中心制备[13]。STZ购自美国Sigma-Aldrich公司,葡萄糖试剂盒由中生北控生物科技股份有限公司提供,INS放射免疫分析试剂盒由上海朗顿生物科技有限公司提供,大鼠白细胞介素6(interleukin-6,IL-6)、CRP、IL-1β、TNF-α和NF-κB ELISA检测试剂盒均购于上海碧云天生物技术有限公司。S10手提式高速分散器(宁波新生生物科技股份有限公司),InfiniteM 200全自动酶标仪(瑞士TECAN集团公司),MH-1微量振荡器(海门市其林贝尔仪器制造有限公司)。
1.2 实验动物分组、造模和处理Wistar大鼠70只,随机选取10只作为正常对照组,饲喂普通饲料;其余60只大鼠饲喂高脂饲料4周后一次性腹腔注射STZ 30 mg·kg-1,建立T2DM模型。正常对照组大鼠给予等体积枸橼酸缓冲液。1周后检测大鼠空腹血糖(fasting blood glucose,FBG),FBG≥7.0 mmol·L-1表示造模成功[15]。将糖尿病模型大鼠随机分为模型组及低、中和高剂量SCP组,每组10只。正常对照组和模型组大鼠给予等体积生理盐水,低、中和高剂量SCP组分别给予25、50和100 mg·kg-1 SCP灌胃,每天1次,连续8周。
1.3 口服葡萄糖耐量实验(oral glucose tolerance test, OGTT)各组大鼠给药治疗8周后进行OGTT,实验前各组大鼠禁食12 h,灌胃给予葡萄糖2.5g·kg-1,分别于灌胃前(0 min)及灌胃后30、60和120 min采集大鼠眼内眦部血样,测定血糖水平,计算血糖曲线下面积(AUC)。公式为:血糖AUC=(C1+C2)×(t1+t2)/2,C1和C2代表不同时间点的血糖浓度,t1和t2代表不同的检测时间点[16]。
1.4 大鼠FBG和INS水平检测末次灌胃给药后,大鼠被禁食12 h,采用乌拉坦(1g·kg-1)进行麻醉,经腹主动脉取血,3500 r·mim-1离心10 min,分离血清于-80℃保存待用。采用葡萄糖氧化酶法检测大鼠FBG水平,放射性免疫分析法检测血清INS水平,具体操作方法参照试剂盒说明书,并计算胰岛素敏感指数(insulin sensitivity index,ISI),ISI= Ln(FBG×INS)-1[15]。
1.5 大鼠血清中炎症因子水平检测采用ELISA法检测大鼠血清中IL-6、CRP、IL-1β、TNF-α和NF-κB水平,具体操作方法参照试剂盒说明书。
1.6 HE染色观察大鼠胰腺组织病理形态表现快速取出上述各组大鼠胰腺组织,并置于10%中性甲醛溶液中固定24 h以上,酒精脱水,二甲苯透明,石蜡包埋,切成厚度为5 μm的切片,行常规HE染色。在普通光学显微镜下观察大鼠胰腺组织病理形态表现。
1.7 统计学分析采用SPSS 22.0统计软件进行统计学分析。各组大鼠血清中FBG、INS和炎症因子水平及ISI经正态性检验呈正态分布,均以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用SNK-q法。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠血清中FBG和INS水平及ISI与正常对照组比较,模型组大鼠FBG水平明显升高(P < 0.01),INS水平和ISI明显降低(P < 0.05或P < 0.01),提示T2DM大鼠建模成功。与模型组比较,各剂量SCP组大鼠血清中FBG水平均明显降低(P < 0.05或P < 0.01),但仍高于正常对照组(P < 0.05或P < 0.01);各剂量SCP组大鼠血清中INS水平和ISI均明显升高(P < 0.05或P < 0.01),但仍低于正常对照组(P < 0.05)。与低剂量SCP组比较,中和高剂量组大鼠FBG水平明显降低(P < 0.05),高剂量SCP组大鼠血清中INS水平明显升高(P < 0.05)。见表 1。
| (n=10, x±s) | |||
| Group | FBG [cB /(mmol·L-1)] | INS [λB / (mU·L-1)] | ISI |
| Normal control | 4.92±0.64 | 32.10±5.00 | -5.13±0.61 |
| Model | 17.30±2.30** | 14.07±1.39** | -5.58±0.66* |
| SCP | |||
| Low dose | 12.41±2.74**△ | 17.71±3.85**△ | -5.46±0.13* |
| Middle dose | 9.76±2.10*△# | 23.55±4.01**△ | -5.35±0.12*△ |
| High dose | 7.34±1.66*△△# | 26.90±4.46*△# | -5.28±0.32△ |
| * P < 0.05, * * P < 0.01 vs normal control group; △ P < 0.05, △△ P < 0.01 vs model group; # P < 0.05 vs low dose of SCP group. | |||
正常对照组、模型组和各剂量SCP组大鼠在葡萄糖注射后30min血糖水平达峰值。在接下来的90min内,正常对照组大鼠血糖水平逐渐下降,在注射120min后基本恢复到正常血糖水平;模型组大鼠血糖水平缓慢下降,其血糖水平与正常对照组、各剂量SCP组比较一直维持在较高水平。此外,模型组大鼠血糖AUC明显高于正常对照组;各剂量SCP组大鼠血糖AUC远低于模型组。见图 1。
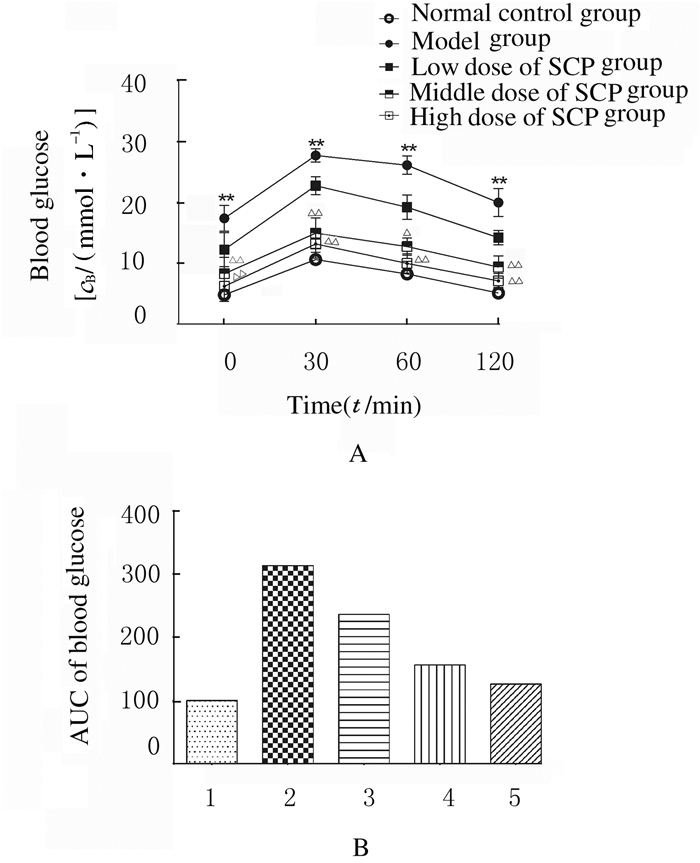
|
| 1:Normal control group; 2:Model group; 3:Low dose of SCP group; 4:Middle dose of SCP group; 5:High dose of SCP group. 图 1 各组大鼠糖耐量水平(A)和血糖AUC(B) Fig. 1 Glucose tolerance levels(A) and AUC of blood glucose(B) of rats in various groups |
|
|
与正常对照组比较,模型组大鼠血清中IL-1β、IL-6、CRP、TNF-α和NF-κB水平均明显升高(P < 0.01),各剂量SCP组大鼠血清中IL-1β和IL-6水平明显升高(P < 0.05或P < 0.01),低、中剂量SCP组大鼠血清中CRP、TNF-α和NF-κB水平明显升高(P < 0.05或P < 0.01),而高剂量SCP组大鼠血清中CRP、TNF-α和NF-κB水平差异无统计学意义(P > 0.05);与模型组比较,各剂量SCP组大鼠血清中IL-1β、IL-6、CRP、TNF-α和NF-κB水平均明显降低(P < 0.05或P < 0.01)。见表 2。
| [n=10, x±s, cB/(μmol·L-1)] | |||||
| Group | IL-1β | IL-6 | CRP | TNF-α | NF-κB |
| Normal control | 42.18±5.88 | 58.41±9.79 | 33.52±4.77 | 150.78±34.65 | 3.52±1.77 |
| Model | 171.12±18.72** | 111.28±18.98** | 50.16±8.00** | 224.27±29.57** | 15.16±2.00** |
| SCP | |||||
| Low dose | 162.97±17.91**△ | 94.33±16.99**△ | 47.36±9.58*△ | 199.78±32.76*△ | 11.36±1.58**△△ |
| Middle dose | 121.28±16.77**△ | 77.55±20.82**△△ | 39.26±9.84*△△ | 170.69±26.97*△△ | 5.63±1.04*△△ |
| High dose | 100.33±9.88**△△ | 74.66±16.99*△△ | 38.63±8.04△△ | 165.08±21.64△ | 4.26±1.84△△ |
| * P < 0.05, * * P < 0.01 vs normal control group; △ P < 0.05, △△ P < 0.01 vs model group. | |||||
正常对照组大鼠胰岛形状规则,呈圆形或椭圆形团索状,边界清晰,胰岛内β细胞分布均匀,排列紧密,胞浆丰富。模型组大鼠胰岛萎缩,结构不清、边缘不整;各剂量SCP组大鼠胰岛内细胞数明显减少,排列稀疏,随着SCP给药剂量的增加,各剂量SCP组胰岛边界逐渐清晰,细胞数目明显增加,细胞形态趋于完好。见图 2(插页三)。
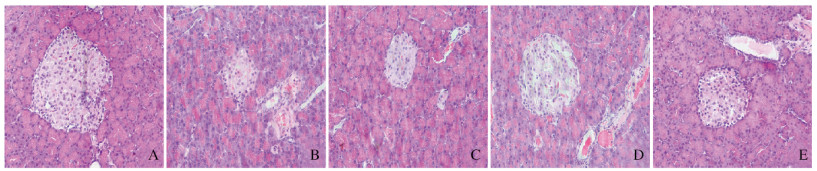
|
| A:Normal control group; B: Model group; C: Low dose of SCP group; D: Middle dose of SCP group; E: High dose of SCP group. 图 2 各组大鼠胰腺组织病理形态表现(HE, ×200) Fig. 2 Pathomorphology of pancreas tissue of rats in various groups (HE, ×200)(seen on page 54 in paragraph) |
|
|
五味子始载于《神农本草经》,具有解除因体液不足和糖尿病引起的口渴的功能。1993年张志军[17]首次明确了五味子对临床糖尿病患者具有改善血糖和糖化血红蛋白(HbA1C)的作用。研究[18]显示:SCP能明显降低糖尿病模型小鼠体质量,减轻烦渴、多尿和高血糖表现,促进肝糖原生成,改善小鼠糖尿病的脂质代谢紊乱。本研究结果也显示:SCP可明显降低高脂饮食联合STZ诱导的T2DM大鼠FBG,升高血清INS水平,提高ISI,显示出对T2DM良好的治疗效果。
T2DM发病的主要环节是IR和胰岛β细胞功能缺陷导致不同程度的INS缺乏。本研究采用高脂高胆固醇饮食喂养大鼠,诱导大鼠出现IR,并联合应用小剂量STZ破坏胰岛β细胞,进而发展为类似于人类T2DM的动物模型。本研究中胰岛病理及OGTT检测结果显示:模型组大鼠胰岛受损、萎缩,葡萄糖耐量明显降低,而SCP治疗可明显改善T2DM大鼠的胰岛病理学变化和葡萄糖耐量,改善了IR。
炎症反应参与T2DM的发生发展过程[19-20]。研究[21-23]显示:在肥胖者脂肪组织中M1型巨噬细胞的浸润,促进IL-6和TNF-α等炎症细胞因子的产生,进而干扰INS信号的转导。巨噬细胞从M2型到M1型的转变对T2DM胰岛功能紊乱起着至关重要的作用[24-25]。此外,M1型巨噬细胞释放的细胞因子刺激胰岛β细胞分泌IL-1β等,从而加速胰岛炎症[26]。因此,直接靶向炎症对血糖控制、β细胞功能和IR均具有有益的影响。研究[27-28]证实:IL-1受体拮抗剂或抗IL-1β的单克隆抗体可降低啮齿类动物和人类的高血糖及炎症细胞因子水平,并减轻巨噬细胞浸润。水杨酸前体药物双水杨酸酯通过抑制NF-κB信号通路,进而降低T2DM患者的血糖、HbA1C和CRP水平[29]。高脂饮食联合STZ诱导的T2DM动物模型通常伴有炎症反应。本研究结果也显示:应用高脂饮食联合小剂量STZ一次性腹腔注射建立的T2DM模型大鼠血清中炎症相关因子IL-6、IL-1β、CRP、TNF-α和NF-κB水平明显升高,而使用SCP治疗后,大鼠血清中IL-6、CRP、IL-1β、TNF-α和NF-κB水平均明显降低,提示SCP治疗能够抑制T2DM大鼠机体的炎症反应,发挥改善IR和修复受损的胰岛β细胞的作用,进而发挥降血糖的作用。
综上所述,SCP可降低T2DM大鼠FBG水平,提高INS水平,改善IR,减轻胰岛损伤。SCP对T2DM有治疗作用,其作用机制可能与抑制机体的炎症反应有关。本研究结果为五味子的进一步开发和利用奠定了理论基础。
| [1] |
HOTAMISLIGIL G S. Inflammation and metabolic disorders[J]. Nature, 2006, 444(7121): 860-867. DOI:10.1038/nature05485 |
| [2] |
DONATH M Y, SHOELSON S E. Type 2 diabetes as an inflammatory disease[J]. Nat Rev Immunol, 2011, 11(2): 98-107. DOI:10.1038/nri2925 |
| [3] |
XI L, XIAO C, BANDSMA R. C-reactive protein impairs hepatic activated protein kinases[J]. Hepatology, 2011, 53(1): 127-135. DOI:10.1002/hep.24011 |
| [4] |
FASSHAUER M, BLVHER M. Adipokines in health and disease[J]. Trends Pharmacol Sci, 2015, 36(7): 461-470. DOI:10.1016/j.tips.2015.04.014 |
| [5] |
RABE K, LEHRKE M, PARHOFER K G, et al. Adipokines and insulin resistance[J]. Mol Med, 2008, 14(11/12): 741-751. |
| [6] |
FENG Y H, CHEN L, LUO Q, et al. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation[J]. Drug Des Devel Ther, 2018, 12: 171-177. DOI:10.2147/DDDT.S157109 |
| [7] |
WANG N, XU T Y, ZHANG X, et al. Improving hyperglycemic effect of FGF-21 is associated with alleviating inflammatory state in diabetes[J]. Int Immunopharmacol, 2018, 56: 301-309. DOI:10.1016/j.intimp.2018.01.048 |
| [8] |
雍履平. 五味子治疗糖尿病功效卓著[J]. 中医杂志, 1998, 39(7): 389. |
| [9] |
JIN D, ZHAO T, FENG W W, et al. Schisandra polysaccharide increased glucose consumption by up-regulating the expression of GLUT-4[J]. Int J Biol Macromol, 2016, 87: 555-562. DOI:10.1016/j.ijbiomac.2016.03.028 |
| [10] |
AN L P, WANG Y P, WANG C M, et al. Protective effect of Schisandrae chinensis oil on pancreatic β-cells in diabetic rats[J]. Endocrine, 2015, 48(3): 818-825. DOI:10.1007/s12020-014-0375-y |
| [11] |
WANG C M, YUAN R S, ZHUANG W Y, et al. Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice[J]. Lipids Health Dis, 2016, 15(1): 195. DOI:10.1186/s12944-016-0358-5 |
| [12] |
PARK H J, LEE S J, SONG Y, et al. Schisandra chinensis prevents alcohol-induced fatty liver disease in rats[J]. J Med Food, 2014, 17(1): 103-110. DOI:10.1089/jmf.2013.2849 |
| [13] |
YUAN R S, TAO X, LIANG S, et al. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress[J]. Biomed Pharmacother, 2018, 99: 537-542. DOI:10.1016/j.biopha.2018.01.079 |
| [14] |
TAO X, LIANG S, CHE J, et al. Antidiabetic activity of acidic polysaccharide from schisandra chinensisin STZ-Induced diabetic mice[J]. Nat Prod Commun, 2019, 14(6). DOI:10.1177/1934578X19850374 |
| [15] |
ZHANG M, LV X Y, LI J, et al. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model[J]. Exp Diabetes Res, 2008, 2008: 704045. |
| [16] |
VEERAPUR V P, PRABHAKAR K R, THIPPESWAMY B S, et al. Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats:A mechanistic study[J]. Food Chem, 2012, 132(1): 186-193. DOI:10.1016/j.foodchem.2011.10.052 |
| [17] |
张志军. 五味子治疗糖尿病的效果[J]. 国外医学:中医中药分册, 1994, 16(3): 27-28. |
| [18] |
NIU J M, XU G Y, JIANG S, et al. In vitro antioxidant activities and anti-diabetic effect of a polysaccharide from Schisandra sphenanthera in rats with type 2 diabetes[J]. Int J Biol Macromol, 2017, 94(Pt A): 154-160. |
| [19] |
AKASH M S, REHMAN K, CHEN S Q. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus[J]. J Cell Biochem, 2013, 114(3): 525-531. DOI:10.1002/jcb.24402 |
| [20] |
KOHLGRUBER A, LYNCH L. Adipose tissue inflammation in the pathogenesis of type 2 diabetes[J]. Curr Diab Rep, 2015, 15(11): 92. DOI:10.1007/s11892-015-0670-x |
| [21] |
HOTAMISLIGIL G S, SHARGILL N S, SPIEGELMAN B M. Adipose expression of tumor necrosis factor-α:direct role in obesity-linked insulin resistance[J]. Science, 1993, 259(5091): 87-91. DOI:10.1126/science.7678183 |
| [22] |
LUMENG C N, BODZIN J L, SALTIEL A R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization[J]. J Clin Invest, 2007, 117(1): 175-184. DOI:10.1172/JCI29881 |
| [23] |
EGUCHI K, NAGAI R. Islet inflammation in type 2 diabetes and physiology[J]. J Clin Invest, 2017, 127(1): 14-23. DOI:10.1172/JCI88877 |
| [24] |
MARCHETTI P. Islet inflammation in type 2 diabetes[J]. Diabetologia, 2016, 59(4): 668-672. DOI:10.1007/s00125-016-3875-x |
| [25] |
EGUCHI K, MANABE I. Macrophages and islet inflammation in type 2 diabetes[J]. Diabetes Obes Metab, 2013, 15(Suppl): 152-158. |
| [26] |
LONTCHI Y, SOBNGWI E, MATSHAT E, et al. Diabetes Mellitus and Inflammation[J]. Curr Diab Rep, 2013, 13(3): 435-444. DOI:10.1007/s11892-013-0375-y |
| [27] |
LARSEN C M, FAULENBACH M, VAAG A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus[J]. N Engl J Med, 2007, 356(15): 1517-1526. DOI:10.1056/NEJMoa065213 |
| [28] |
SLOAN-LANCASTER J, ABU-BADDADE, POLZER J, et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes[J]. Diabetes Care, 2013, 36(8): 2239-2246. DOI:10.2337/dc12-1835 |
| [29] |
GOLDFINE A B, FONSECA V, JABLONSKI K A, et al. Salicylate (salsalate) in patients with type 2 diabetes:A randomized trial[J]. Ann Intern Med, 2013, 159(1): 1-12. DOI:10.7326/0003-4819-159-1-201307020-00003 |
 2020, Vol. 46
2020, Vol. 46


