扩展功能
文章信息
- 李松岩, 林冬静, 于洋, 刘师兵, 徐路, 孙奇, 徐冶
- LI Songyan, LIN Dongjing, YU Yang, LIU Shibing, XU Lu, SUN Qi, XU Ye
- 沉默信息调节因子3对白藜芦醇诱导的人卵巢癌SKOV3细胞凋亡的影响
- Effect of silencing sirtuin 3 on resveratrol-induced apoptosis of human ovarian cancer SKOV3 cells
- 吉林大学学报(医学版), 2020, 46(01): 45-49
- Journal of Jilin University (Medicine Edition), 2020, 46(01): 45-49
- 10.13481/j.1671-587x.20200108
-
文章历史
- 收稿日期: 2019-03-18
2. 吉林医药学院科研实验室, 吉林 吉林 132013
2. Scientific Research Laboratory, Jilin Medical University, Jilin 132013, China
卵巢癌是女性生殖器官常见的恶性肿瘤之一[1-2],卵巢癌的发病率和死亡率占各类妇科恶性肿瘤的首位[3-5]。白藜芦醇(resveratrol,Res)是一种天然的多酚类化合物,其抗肿瘤作用已经得到确认[6-7]。沉默信息调节因子3 (silent mating type information regulation 3,Sirt3)是一种依赖于烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD)的Ⅲ类去乙酰化酶,其在线粒体的适应性反应中起调节作用[8]。研究[9-11]表明:Sirt3影响肿瘤的发生发展,当Sirt3基因缺失时,可以增加细胞的糖酵解过程,促进肿瘤的生长。Sirt3也可影响基因组,抑制癌症相关代谢和影响肿瘤微环境,在癌症的发展过程中发挥不同的作用[12-13]。研究[14-15]显示:Res可以通过调节Sirt3表达进而调控细胞的功能。为了明确Res对SKOV3细胞的促凋亡作用,本研究采用Sirt3抑制剂3-(1H-1, 2, 3-三唑-4-基)吡啶[3-(1H-1, 2, 3-triazol-4-yl) pyridine, 3-TYP]抑制Sirt3表达后,探讨Sirt3在Res诱导SKOV3细胞凋亡中的作用机制,为进一步研究Res的抗肿瘤作用及其在临床中的应用提供理论依据。
1 材料与方法 1.1 细胞、主要试剂和仪器人卵巢癌SKOV3细胞由吉林医药学院肿瘤靶向治疗与转化医学重点实验室保存,用含有10%胎牛血清的RPMI 1640培养液培养。Res购自上海阿拉丁生化科技股份有限公司,溶于DMSO中,4℃保存。MTT和DMSO购自Sigma公司,Hoechst33342荧光染料购自北京鼎国公司,活性氧(ROS)荧光探针-DHE购自北京索莱宝生物科技有限公司,兔抗人B细胞淋巴瘤/白血病-2(Bcl-2)、Bcl-2相关X蛋白(Bax)、半胱氨酸天冬氨酸蛋白水解酶3(cleaved caspase-3)和β-actin单克隆抗体及含有辣根过氧化物酶的羊抗兔二抗均购自长春德尔塔生物有限公司,3-TYP购自上海陶术生物科技有限公司。恒温二氧化碳细胞培养箱(日本SANYO公司),电子分析天平(瑞士梅特勒托利多公司),超净工作台(北京东联哈尔公司),高速台式冷冻离心机(德国Hermle公司),倒置光学显微镜(日本Leica公司),水浴锅(上海精宏实验设备有限公司),Model-680型酶标仪、蛋白电泳仪和蛋白转印仪(美国Bio-rad公司),脱色摇床(北京六一医学仪器厂),超声细胞粉碎仪(宁波新芝生物科技有限公司),高温高压蒸汽灭菌锅(上海博讯实业有限公司),数码凝胶成像系统(上海天能科技有限公司)。
1.2 MTT法检测SKOV3细胞存活率取对数生长期的SKOV3细胞以每孔5×104个细胞接种到96孔板中。Res用DMSO溶解后,用不同浓度(0、2.5、5.0、10.0、20.0、40.0和80.0 mg·L-1) Res处理细胞,培养24h后每孔加入MTT(5 mg·L-1)20 μL,4 h后弃去含有MTT的培养液,每孔加入DMSO 150μL。用酶标仪在490nm处检测每孔吸光度(A)值。每次检测重复3次。细胞存活率=(实验组A值-空白组A值)/(对照组A值-空白组A值)×100%。
1.3 MTT法检测各组细胞的增殖抑制率取对数生长期的SKOV3细胞以每孔5×104个细胞接种到96孔板中。3-TYP用DMSO溶解后,稀释成相应浓度。将细胞分为对照组、3-TYP(50μmol·L-1)组、Res(30 mg·L-1)组和3-TYP+Res组,孵育24 h后,每孔加入MTT20 μL,4 h后弃去培养液,加入DMSO 150 μL。用酶标仪检测每孔A值,每次检测重复3次。细胞增殖抑制率=(1-实验组A值/空白组A值)×100%。
1.4 Hoechst 33342染色观察SKOV3细胞核形态SKOV3细胞以1×105mL-1密度接种于24孔板,分组同上,24 h后用PBS洗涤3次,每孔加入4%多聚甲醛200 μL固定25min,用PBS洗涤3次,加入200 μLHoechst 33342荧光染料染色5 min,PBS洗涤3次,用甘油封片,在激光共聚焦显微镜下观察SKOV3细胞核形态。
1.5 ROS探针检测SKOV3细胞中ROS水平细胞分组同上,培养24h后,弃去培养液,加入PBS 500mL洗涤3次,加入用RPIM1640培养液稀释的ROS探针(1:2500)孵育20min,激光共聚焦显微镜下观察探针显色情况。以红色荧光强度表示ROS水平。
1.6 Western blotting法检测SKOV3细胞中相关蛋白表达水平细胞分组同上,培养24h, 收集各组细胞,用PBS洗涤2次,加入蛋白裂解液RIPA 150μL,用超声细胞破碎仪破碎细胞后,冰上放置30min,台式高速冷冻离心机离心,收集上清。BCA法测定蛋白浓度。SDS-PAGE电泳结束后,将蛋白转移至PVDF膜上,5%脱脂奶粉封闭1.5h,PBST洗涤3次,用Sirt3抗体(1:1 000)、Bcl-2抗体(1:2 000)、Bax抗体(1:2 000)、cleavecaspase-3抗体(1:500)和β-actin抗体(1:1 000)4℃孵育过夜。次日PBST洗涤3次,用HRP标记的二抗(1:5 000)室温孵育1.5 h,PBST洗涤5次,ECL显色液显色。以β-actin作为内参照,采用Quantity One软件对结果进行分析处理,并计算目的蛋白相对表达水平。
1.7 统计学分析采用SPSS 18.0统计软件进行统计学分析。各组SKOV3细胞存活率,Sirt3、Bcl-2、Bax和cleaved caspase-3蛋白表达水平均以x±s表示,多组间样本均数比较采用单因素方差,均数间多重比较采用LSD-t检验。以P < 0.05为差异有统计学意义。
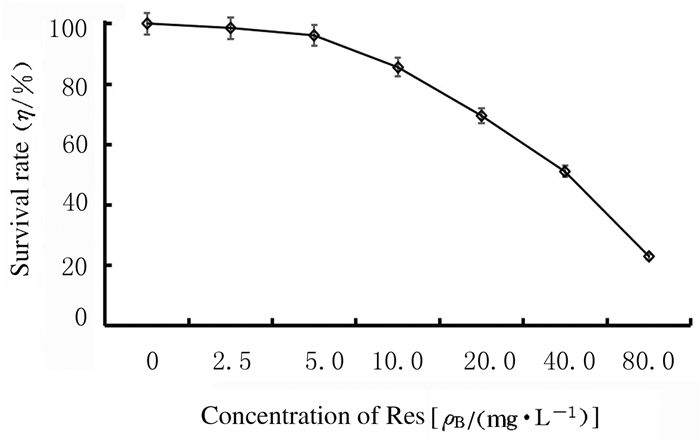
2 结果 2.1 MTT法检测SKOV3细胞存活率不同浓度Res处理SKOV3细胞24h后,随着Res浓度的增高,细胞存活率明显降低,Res的半数抑制浓度(IC50)为42.73 mg·L-1。见图 1。
|
| 图 1 MTT法检测SKOV3细胞存活率 Fig. 1 Survival rates of SKOV3 cells detected by MTT method |
|
|
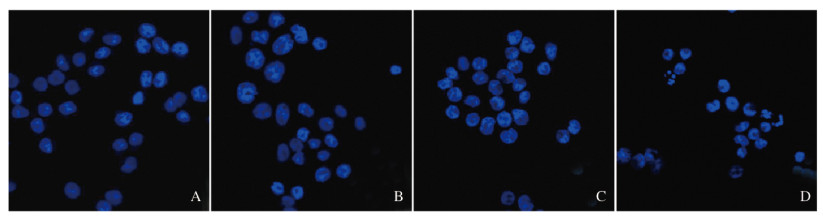
与对照组(0.00%±1.37%)比较,Res组和3-TYP+Res组细胞增殖抑制率(0.26%±0.06%和0.47%±0.09%)明显升高(P < 0.05);与Res组比较,3-TYP+Res组细胞增殖抑制率进一步升高(P < 0.05)。应用Hoechst 33342染色,在激光共聚焦显微镜下观察SKOV3细胞核形态表现:3-TYP+Res组细胞核出现固缩、染色增强、核碎裂增多,其余各组只有个别细胞出现类似现象。见图 2(插页三)。
|
| A:Control group;B:3-TYP group;C:Res group;D:3-TYP+Res group. 图 2 各组SKOV3细胞核形态表现(Hoechst 33342, ×400) Fig. 2 Morphology of SKOV3 cell nuclei in various groups (Hoechst 33342, ×400) |
|
|
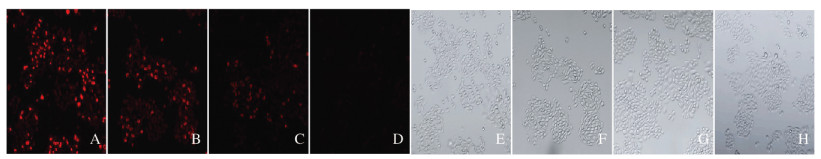
以ROS荧光探针-DHE进行染色,红色荧光强度代表ROS水平,激光共聚焦显微镜下观察。与对照组比较,3-TYP组细胞红色荧光无明显变化,Res组和3-TYP+Res组细胞红色荧光明显减少。见图 3(插页三)。
|
| A-D:ROS;E-H:Bright field; A, E:Control group;B, F:3-TYP group;C, G:Res group;D, H:3-TYP+Res group. 图 3 激光共聚焦显微镜观察各组SKOV3细胞中荧光强度(×400) Fig. 3 Fluorescence intensities in SKOV3 cells in various groups detected by laser confocal microscope(×400) |
|
|
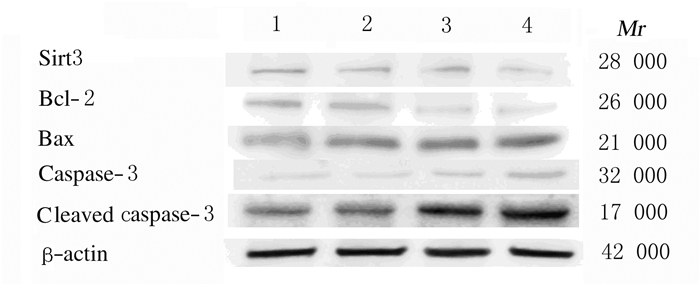
与对照组比较,3-TYP组细胞中Sirt3蛋白表达水平明显降低(t=-5.37,P < 0.05),Bcl-2、Bax和cleaved caspase-3蛋白表达水平无明显变化(P > 0.05);Res组细胞中Sirt3和Bcl-2蛋白表达水平明显降低(t=-8.71,P < 0.05;t=-4.67,P < 0.05),Bax和cleaved caspase-3蛋白表达水平明显升高(t=3.64,P < 0.05;t=5.72,P < 0.05);与Res组比较,3-TYP+Res组细胞中Bax和cleaved caspase-3蛋白表达水平明显升高(t=8.47,P < 0.05;t=4.29,P < 0.05),Bcl-2和Sirt3蛋白表达水平明显降低(t=-10.52,P < 0.05;t=-7.61,P < 0.05)。见图 4和表 1。
|
| Lane 1:Control group;Lane 2:3-TYP group;Lane 3:Res group;Lane 4:3-TYP+Res group. 图 4 Western blotting法检测各组SKOV3细胞中凋亡相关蛋白表达电泳图 Fig. 4 Electrophoregram of expressions of apoptosis-related proteins in SKOV3 cells in various groups detected by Western blotting method |
|
|
| (n=3, x±s) | ||||
| Group | Sirt3 | Bcl-2 | Bax | Cleaved caspase-3 |
| Control | 0.412±0.024 | 0.797±0.103 | 0.462±0.037 | 0.452±0.091 |
| 3-TYP | 0.196±0.014* | 0.632±0.021 | 0.507±0.157 | 0.519±0.094 |
| Res | 0.318±0.027 | 0.433±0.125* | 0.746±0.114* | 0.752±0.172* |
| 3-TYP+Res | 0.207±0.059*△ | 0.361±0.168*△ | 0.869±0.207*△ | 0.963±0.211*△ |
| * P < 0.05 compared with control group;△ P < 0.05 compared with Res group. | ||||
细胞凋亡是细胞程序性死亡的过程,其对一些妇科肿瘤的发生发展均可产生影响[16]。诱导肿瘤细胞凋亡是最理想的治疗肿瘤的策略[17]。已有研究[18-19]证明:Res能够诱导多种肿瘤细胞凋亡,包括白血病、结肠癌、乳腺癌、前列腺癌、肝癌和食道癌等。本研究结果表明:Res作用SKOV3细胞后,细胞存活率下降,且呈浓度依赖性,与已报道结果一致[9]。为进一步研究Res诱导SKOV3细胞凋亡的机制,本文作者用Sirt3抑制剂3-TYP作用于SKOV3细胞,进一步观察Res对SKOV3细胞影响的结果显示:与Res组比较,3-TYP+Res组SKOV3细胞生长抑制率明显升高,并且Hoechst 33342染色显示细胞核染色增强、核碎裂明显增多,表明应用3-TYP抑制Sirt3表达后,可增强Res对SKOV3细胞的抑制作用。研究[20]表明:线粒体中的Sirt3可以激活超氧化物歧化酶含锰金属辅基(MnSOD)和异柠檬酸脱氢酶2(IDH2),防止ROS在线粒体中积累。而Res又具有明显的抗氧化、抗自由基和抗癌等功效[21],因此Sirt3可能通过ROS影响Res对SKOV3的作用。本研究结果显示:与对照组比较,Res组SKOV3细胞中ROS水平降低,而3-TYP+Res组SKOV3细胞中ROS水平进一步降低,表明Sirt3通过细胞中ROS影响Res对SKOV3细胞的抑制作用。细胞凋亡的线粒体途径中,Bcl-2家族起关键作用[20, 22]。本研究结果显示:与Res组比较,3-TYP和Res联合作用SKOV3细胞24h后,凋亡相关蛋白Bax和cleavedcaspase-3蛋白表达水平明显升高,Bcl-2和Sirt3蛋白表达水平明显降低,可能是抑制Sirt3后导致线粒体内ROS改变,进而使Res抗氧化能力的增强,SKOV3细胞内凋亡途径被激活。
综上所述,Res诱导卵巢癌SKOV3细胞凋亡的作用的机制可能是通过内源性线粒体途径激活Bcl-2家族,进而激活caspase-3诱导细胞凋亡。Res可能通过Sirt3-ROS-Bcl-2通路诱导SKOV3细胞凋亡。Sirt3能够协同Res增强细胞凋亡作用,但其最终能否成为治疗肿瘤的一个新的途径还需进一步研究。
| [1] |
YO SHIDA H. ER stress and diseases[J]. FEBS J, 2007, 274(3): 630-658. DOI:10.1111/j.1742-4658.2007.05639.x |
| [2] |
WALTER P, RON D. The unfolded protein response:from stress pathway to homeostatic regulation[J]. Science, 2011, 334(6059): 1081-1086. DOI:10.1126/science.1209038 |
| [3] |
MEDIGESHI G R, LANCASTER A M, HIRSCH A J, et al. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis[J]. J Virol, 2007, 81(20): 10849-10860. DOI:10.1128/JVI.01151-07 |
| [4] |
杨念念, 严亚琼, 郑荣寿, 等. 中国2009年卵巢癌发病与死亡分析[J]. 中国肿瘤, 2013, 22(8): 617-621. |
| [5] |
卢淮武, 王东雁, 林仲秋. 《2015美国肿瘤综合协作网卵巢癌输卵管癌原发性腹膜癌临床实践指南(第1版)》解读[J]. 中国实用妇科与产科杂志, 2015, 31(5): 378-384. |
| [6] |
WOZNIAK K, BLASIAK J. Recognition and repair of DNA-cisplatinadducts[J]. Acta Biochim Pol, 2002, 49(3): 583-596. DOI:10.18388/abp.2002_3768 |
| [7] |
REEDIJK J. New clues for platinum antitumor chemistry:kinetically controlled metal binding to DNA[J]. Proc Natl Acad Sci U S A, 2003, 100(7): 3611-3616. DOI:10.1073/pnas.0737293100 |
| [8] |
TORRENS-MAS M, OLIVER J, ROCA P, et al. SIRT3:Oncogene and tumor suppressor in cancer[J]. Cancers (Basel), 2017, 9(7): E90. |
| [9] |
ANSARI A, RAHMAN M S, SAHA S K, et al. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease[J]. Aging Cell, 2017, 16(1): 4-16. |
| [10] |
PALMIROTTA R, CIVES M, DELLAMORTE D, et al. Sirtuins and cancer:Role in the epithelial-mesenchymal transition[J]. Oxidative Med Cell Longevity, 2016, 13(8): 1-9. |
| [11] |
TORRENSMAS M, PONS D G, SASTRE-SERRA J, et al. SIRT3 silencing sensitizes breast cancer cells to cytotoxic treatments through an increment in ROS production[J]. J Cell Biochem, 2017, 118(2): 397-406. DOI:10.1002/jcb.25653 |
| [12] |
GEORGE J, NIHAL M, SINGH C K, et al. Pro-proliferative function of mitochondrial sirtuin deacetylase SIRT3 in human melanoma[J]. J Invest Dermatol, 2016, 136(4): 809-818. DOI:10.1016/j.jid.2015.12.026 |
| [13] |
LIU Y, LIU Y L, CHENG W, et al. The expression of SIRT3 in primary hepatocellular carcinoma and the mechanism of its tumor suppressing effects[J]. Eur Rev Med Pharmacol Sci, 2017, 21(5): 978-998. |
| [14] |
MICHISHITA E, PARK J Y, BURNESKIS J M, et al. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins[J]. Mol Biol Cell, 2005, 16(10): 4623-4635. DOI:10.1091/mbc.e05-01-0033 |
| [15] |
LOMBARD D B, ALT F W, CHENG H L, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation[J]. Mol Cell Biol, 2007, 27(24): 8807-8814. DOI:10.1128/MCB.01636-07 |
| [16] |
HEBERT A S, DITTENHAFER-REED K E, YU W, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome[J]. Mol Cell, 2013, 49(1): 186-199. DOI:10.1016/j.molcel.2012.10.024 |
| [17] |
NALEPA G, ROLFE M, HARPER J W. Drug discovery in the ubiquitin-proteasome system[J]. Nat Rev Drug Discov, 2006, 5(7): 596-613. DOI:10.1038/nrd2056 |
| [18] |
ROSENBERG B, VANCAMP L, KRIGAS T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode[J]. Nature, 1965, 205: 698-699. DOI:10.1038/205698a0 |
| [19] |
AGGARWAL B B, BHARDWAJ A, AGGARWAL R S, et al. Role of resveratrol in prevention and therapy of cancer:preclinical and clinical studies[J]. Anticancer Res, 2004, 24(5A): 2783-2791. |
| [20] |
OLTVAI Z N, MILLIMAN C L, KORSMEYER S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death[J]. Cell, 1993, 74(4): 609-619. DOI:10.1016/0092-8674(93)90509-O |
| [21] |
SELVARAJ S, SUN Y Y, SUKUMARAN P, et al. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway[J]. Mol Carcinog, 2016, 55(5): 818-831. DOI:10.1002/mc.22324 |
| [22] |
SUNDARESAN N R, SAMANT S A, PILLAI V B, et al. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70[J]. Mol Cell Biol, 2008, 28(20): 6384-6401. DOI:10.1128/MCB.00426-08 |
 2020, Vol. 46
2020, Vol. 46


