扩展功能
文章信息
- 王建军, 刘亚楠, 王建辉, 刘燕, 李颖, 王瑞雪, 杨秀红
- WANG Jianjun, LIU Yanan, WANG Jianhui, LIU Yan, LI Ying, WANG Ruixue, YANG Xiuhong
- 二乙酰胺三氮脒对肢体缺血再灌注模型小鼠肾损伤的保护作用及其机制
- Protective effect of diminazene on kidney injury of limb ischemia-reperfusion model mice and its mechanism
- 吉林大学学报(医学版), 2020, 46(01): 14-19
- Journal of Jilin University (Medicine Edition), 2020, 46(01): 14-19
- 10.13481/j.1671-587x.20200103
-
文章历史
- 收稿日期: 2019-03-26
2. 华北理工大学附属医院重症医学科, 河北 唐山 063000;
3. 河北省唐山市慢性病重点实验室, 河北 唐山 063000
2. Department of Intersive Medicine Unit, Affiliated Hospital, North China University of Science and Technology, Tangshan 063000, China;
3. Key Laboratory of Chronic Diseases, Tangshan City, Hebei Province, Tangshan 063000, China
肢体长时间缺血后突然恢复其血流灌注,除肢体本身受到严重损伤外,还会出现更为严重的远隔组织器官损伤,如肾脏、心脏、肺脏和肠道都会出现或序贯性出现不同程度损伤,引起多脏器功能障碍,严重时甚至出现休克和多脏器功能衰竭,其发病机制尚不十分明确。研究[1]表明:肢体缺血后再灌注(limb ischemia-reperfusion,LIR)后远隔组织器官损伤与局部肾素血管紧张素系统(renin-angiotensin system,RAS)表达失衡有关。本文作者通过研究血管紧张素转换酶2(angiotensin converting enzyme 2,ACE2)激动剂二乙酰胺三氮脒(diminazene,DIZE)对小鼠LIR后肾损伤指标和肾局部组织RAS系统中血管紧张素Ⅱ(angiotensin Ⅱ,AngⅡ)和血管紧张素(1-7) [angiotensin(1-7),Ang(1-7)]水平及其相应受体血管紧张素Ⅱ1型受体(angiotensin Ⅱ type-1 receptor,AT1R)和Mas受体(Mas receptor,MasR)蛋白表达的影响,探讨DIZE对肾损伤的保护作用及其可能机制,为LIR后器官保护提供新的实验依据和策略。
1 材料与方法 1.1 主要试剂和仪器蛋白提取试剂盒、蛋白浓度测定试剂盒、Western blotting蛋白Marker、ECL发光剂、定影剂和显影剂均为碧云天生物技术公司产品,ELISA-Ang(1-7)试剂盒和ELISA-AngⅡ试剂盒均购自武汉优尔生科技股份有限公司,AT1R一抗试剂盒购自美国Santa Cruz公司,MasR一抗购自以色列Alomone Labs公司,DIZE购自美国Sigma公司。套扎枪(M-M-0084),江苏金坛医疗器械有限公司;酶标仪(Model 680), 美国Bio-RAD公司;电泳仪(ECP3000), 北京六一仪器厂;低温高速离心机(Centrifuge 5804R), 德国Eppendorf公司;SM2400平推式切片机,德国Leica公司。
1.2 实验动物ICR小鼠由中国医学科学院实验动物研究所提供,在华北理工大学实验动物中心SPF级动物房采用SPF级基础饲料和繁殖饲料进行繁育和饲养,小鼠自由进食和饮水。选取8周龄雄性ICR小鼠18只,随机分为对照组、LIR组和LIR后DIZE治疗(LIR+DIZE)组,每组6只。
1.3 LIR模型制备腹腔注射水合氯醛(3 mg·kg-1)麻醉小鼠,制备LIR模型:在双后肢根部行橡皮套结扎术,使双后肢缺血2h,随后用手术剪剪断橡胶圈,并对小鼠双侧后肢按摩,进行血流再灌注4 h。LIR组和LIR+DIZE组小鼠采用常规方法复制小鼠LIR模型,LIR+DIZE组小鼠于LIR前皮下注射DIZE(10 mg·kg-1·d-1)预处理14 d。摘除眼球取血处死小鼠,留取血浆和双侧肾脏,分别在-80℃冰箱和甲醛固定液中保存备用。
1.4 HE染色观察小鼠肾组织病理形态表现将小鼠一侧肾脏浸于10%甲醛中充分固定后,按常规方法脱水、包埋、切片、HE染色,显微镜下观察小鼠肾组织病理形态表现。参照文献[2]方法进行病理损伤评分, 即每张切片在皮质区随机观察10个无重叠视野(×200),每个视野选取50个肾小管进行评分:正常肾小管为0分,刷状缘消失为2分,肾小管明显扩张、细胞扁平为1分,间质水肿1分,细胞质空泡2分,肾小管细胞坏死2分,形成管型或碎片为2分。每个肾小管病理损伤评分总计为10分,每个视野最高评分为500分。
1.5 小鼠肾功能测定血清离心取上清,打开生化仪电源及系统软件,设置测定小鼠血清中尿素和血肌酐(Scr)水平,反应温度37℃,使用与试剂配套的校准溶液进行定标,先将待测的样本依次放入样本位中,然后启动测定程序,进行检测。
1.6 ELISA法检测小鼠肾组织中AngⅡ和Ang(1-7)水平应用竞争性抑制酶联免疫分析法测定小鼠肾组织中AngⅡ和Ang(1-7)水平。将肾组织匀浆、离心,取上清,按试剂盒说明操作,用酶标仪在450nm波长下测定吸光度(A)值,根据标准曲线计算AngⅡ和Ang(1-7)水平。
1.7 蛋白免疫印迹(Western blotting)法检测小鼠肾组织中AT1R和MasR蛋白表达水平按照蛋白提取试剂盒说明提取蛋白。取肾组织100 mg放入玻璃匀浆器,加入1 mL蛋白裂解液匀浆,12 000r·min-1离心30 min,留取上清。用BCA法测定蛋白浓度,用裂解液将各蛋白配成浓度为3 mg·L-1,取10 μL蛋白样品与5×上样缓冲液混匀,煮沸10 min后SDS-PAGE电泳,转硝酸纤维素膜45 min,5%BSA室温封闭1 h,一抗孵育过夜,TBST洗膜3次,每次10 min,二抗37℃温箱孵育1 h,TBST洗膜3次,每次10 min。ECL发光试剂均匀铺于膜上孵育2 min,曝光后显影、定影。用ImageJ图像分析软件分析处理条带灰度值,计算蛋白相对表达水平,以目的条带与内参灰度值比值表示目的蛋白相对表达水平。
1.8 统计学分析采用SPSS17.0统计软件进行统计学分析。肾病理损伤评分,血清Urea和Scr水平,肾组织中AngⅡ和Ang(1-7)水平及AT1R和MasR蛋白表达水平均以x±s表示,多组间样本均数比较采用单因素方差分析(One-way ANOVA),组间两两比较采用LSD法。以P<0.05为差异有统计学意义。
2 结果 2.1 各组小鼠肾组织病理形态表现及病理损伤评分光镜下观察,与对照组比较,LIR组小鼠肾组织中肾小球毛细血管扩张充血,炎细胞浸润,间质水肿,肾小管上皮细胞坏死,蛋白质管型较为常见,肾组织病理损伤评分明显升高(P<0.05)。与LIR组比较,LIR+DIZE组小鼠肾组织中偶见肾小球毛细血管扩张充血、炎细胞浸润、间质水肿和肾小管上皮脱落等病理表现,肾组织病理损伤评分明显降低(P<0.05)。见图 1(插页一)和表 1。
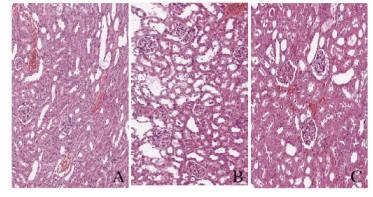
|
| A: Control group; B: LIR group: C: LIR DIZE group. 图 1 各组小鼠肾组织病理形态表现(HE, ×200) Fig. 1 Pathomorphology of kidney tissue of mice in various groups (HE, ×200) |
|
|
| (n=6, x±s) | |
| Group | Pathological injury score |
| Control | 3.50±1.64 |
| LIR | 283.00±21.35* |
| LIR+DIZE | 186.67±18.38*△ |
| * P<0.05 vs control group; △ P<0.05 vs LIR group. | |
与对照组比较,LIR组和LIR+DIZE组小鼠血清中尿素和Scr水平明显升高(P<0.05);与LIR组比较,LIR+DIZE组小鼠血清中尿素和Scr水平明显降低(P<0.05)。见表 2。
| (n=6, x±s) | ||
| Group | Serum urea[cB/(mmol·L-1)] | Scr [cB/(μmol·L-1)] |
| Control | 8.59±0.72 | 2.68±1.02 |
| LIR | 33.87±7.91* | 71.24±15.22* |
| LIR+DIZE | 23.60±4.52*△ | 41.91±7.04*△ |
| * P<0.05 vs control group; △ P<0.05 vs LIR group. | ||
与对照组比较,LIR组小鼠肾组织中AngⅡ和Ang(1-7)水平及AngⅡ/Ang(1-7)比值明显升高(P<0.05);与LIR组比较,LIR+DIZE组小鼠肾组织AngⅡ水平明显降低(P<0.05),Ang(1-7)水平明显升高(P<0.05),AngⅡ/Ang(1-7)比值明显降低(P<0.05)。见表 3。
| (n=6, x±s) | |||
| Group | AngⅡ [ρB/(ng·L-1)] |
Ang(1-7 )[ρB/(ng·L-1)] |
AngⅡ/Ang(1-7) |
| Control | 15.67±1.39 | 27.39±3.69 | 0.58±0.08 |
| LIR | 58.38±5.04* | 66.46±5.66* | 0.87±0.09* |
| LIR+DIZE | 46.67±5.83*△ | 77.67±6.57*△ | 0.65±0.03*△ |
| * P<0.05 vs control group; △ P<0.05 vs LIR group. | |||
与对照组比较,LIR组小鼠肾组织中AT1R蛋白表达水平明显降低(P<0.05),MasR蛋白表达水平明显升高(P<0.05),AT1R/MasR比值降低(P<0.05);与LIR组比较,LIR+DIZE组小鼠肾组织中AT1R和MasR蛋白表达水平明显升高(P<0.05),AT1R/MasR比值也明显升高(P<0.05)。见图 2和表 4。
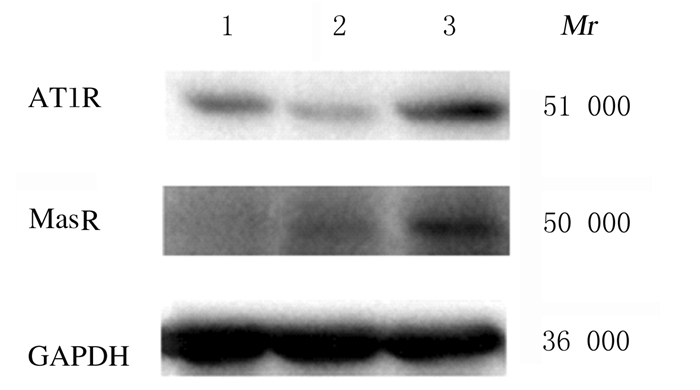
|
| Lane 1:Control group; Lane 2:LIR group; Lane 3:LIR+DIZE group 图 2 各组小鼠肾组织中AT1R和MasR蛋白表达电泳图 Fig. 2 Electrophoregram of AT1R and MasR proteins in kidney tissue of mice in various groups |
|
|
| (n=6, x±s) | |||
| Group | AT1R | MasR | AT1R/MasR |
| Control | 0.30±0.03 | 0.40±0.03 | 0.75±0.05 |
| LIR | 0.20±0.01* | 0.60±0.04* | 0.30±0.04* |
| LIR+DIZE | 0.90±0.10*△ | 1.10±0.2*△ | 0.82±0.03*△ |
| * P<0.05 vs control group; △ P<0.05 vs LIR group. | |||
LIR可引起远隔器官如心脏、肺脏和肾脏等的损伤,其机制十分复杂。本课题组前期研究[1-2]显示:LIR后急性肾和肺损伤与器官局部组织RAS失衡有关。器官局部RAS由两条作用相反的通路组成,一条是血管紧张素转换酶(ACE)-AngⅡ-AT1R轴,另一条是ACE2-Ang(1-7)-MasR轴,两条轴的作用相反,其表达失衡可能是引起器官损伤的一个重要原因。在各种病理情况下ACE-AngⅡ-AT1R过表达可通过炎症和过氧化等机制造成器官损害,而ACE2-Ang(1-7)-MasR轴的作用正好与其相反,对器官具有保护作用。
有研究[3]表明:传统使用的广谱抗血液原虫药DIZE可以激活RAS中的ACE2,兴奋全身的ACE2-Ang(1-7)-MasR轴,从而起到器官保护作用。FOUREAUX等[4]研究发现:DIZE对ACE2-Ang(1-7)-MasR轴的干预,可能成为治疗青光眼的潜在策略。也有研究[5]表明:中枢给予DIZE可明显减少脑梗死的面积,其效果与外周给予Ang(1-7)激活ACE2-Ang(1-7)-MasR轴的效果相似。也有研究[6]显示:DIZE通过影响ACE2-Ang(1-7)-MasR轴的表达,改善阿尔茨海默病大鼠模型的认知障碍。QI等[7]研究发现:DIZE可以通过提高ACE2表达,改变心脏RAS水平,增加心脏祖细胞和循环内皮祖细胞,减少心肌梗死区域巨噬细胞浸润,调控致炎因子,减少心脏诱导的梗死区域的细胞凋亡,从而治疗心肌梗死诱导的左心功能不全。有研究[8]显示:DIZE可通过激活心脏ACE2轴增强心肌保护作用,比依那普利更能改善急性心肌梗死后的心肌功能。CHEN等[9]研究表明:DIZE能明显降低肿瘤坏死因子α和白细胞介素6等炎症因子的水平,激活ACE2和AT1R, 从而减少心肌梗死面积。DIZE还以通过激动ACE2-Ang(1-7)-MasR轴防止肝脏纤维化[10]。在LIR所致肺损伤小鼠模型中,DIZE可通过改善RAS两轴的失衡发挥肺保护作用[11]。MALEK等[12]发现:应用DIZE处理肾缺血再灌注后的大鼠,能明显改善再灌注后的肝肾功能。DIZE还可以减少活性氧(ROS)产物和三磷酸吡啶核苷酸(NADPH)氧化酶的表达,通过抗氧化和提高亚硝酸盐水平而发挥对肾脏的保护作用[13]。研究[14-15]表明:在糖尿病大鼠模型中,DIZE通过影响RAS相关因子表达,保护肾功能,预防糖尿病肾病的发生。KANGUSSU等[16]研究表明:DIZE通过激动ACE2改善高血压所致肾脏病变。也有研究[17]显示:DIZE可以降低体外培养的人视网膜色素上皮细胞AngⅡ和AT1R的表达和促进Ang(1-7)表达,从而减轻脂多糖诱导的炎症反应。研究[18-19]表明:RAS可影响肝脂肪代谢,ACE2-Ang(1-7)-MasR轴的高表达有预防和治疗脂肪肝的作用。但也有研究[20]证实:DIZE不能提高内皮的ACE2活性,从而不能在局部组织产生足量的Ang(1-7)来影响血管的紧张性,而是通过抑制ACE的活性发挥作用。
研究[21]显示:LIR后肾组织局部出现RAS稳态失衡。ACE2激动剂DIZE是否可通过激活ACE2改善RAS失衡而发挥对肾损伤的保护作用,目前尚未见报道。本实验中,LIR模型小鼠局部肾组织AngⅡ/Ang(1-7)和AT1R/MasR比值均出现失衡,血清中尿素和Scr水平均升高,病理结果显示小鼠有不同程度肾损伤;应用DIZE预处理后,RAS失衡明显改善,小鼠肾病理损伤明显减轻,而且Urea和Scr水平明显降低。因此本文作者推测:作为ACE2激动剂,DIZE可能通过激动ACE2,大量水解AngⅡ生成Ang(1-7),降低AngⅡ水平,同时增加组织中Ang(1-7)的水平,上调MasR表达,改善RAS失衡状态而使肾损伤减轻。
综上所述,AngⅡ/Ang(1-7)及AT1R/MasR表达失衡在LIR后急性肾损伤发生中起重要作用。ACE2激动剂DIZE可能通过改善RAS稳态失衡发挥对肾脏的保护作用。
| [1] |
王建军, 杨秀红, 邓巍, 等. 小鼠止血带休克后肾组织ACE/ACE2表达变化及意义[J]. 中国病理生理杂志, 2011, 27(12): 2399-2402. DOI:10.3969/j.issn.1000-4718.2011.12.031 |
| [2] |
王建辉, 张伟, 刘燕, 等. 小鼠肢体缺血再灌注后肾组织AT1和Mas受体蛋白差异性表达与肾损伤的关系[J]. 解放军医学杂志, 2016, 41(3): 184-188. |
| [3] |
DASGUPTA C, ZHANG L B. Angiotensin Ⅱ receptors and drug discovery in cardiovascular disease[J]. Drug Discov Today, 2011, 16(1/2): 22-34. |
| [4] |
FOUREAUX G, NOGUEIRA J C, NOGUEIRA B S, et al. antiglaucomatous effects of the activation of intrinsic angiotensin-converting enzyme 2[J]. Invest Ophthalmol Vis Sci, 2013, 54(6): 4296-4306. DOI:10.1167/iovs.12-11427 |
| [5] |
MECCA A P, REGENHARDT R W, ÓCONNOR T, et al. Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke[J]. Exp Physiol, 2011, 96(10): 1084-1096. DOI:10.1113/expphysiol.2011.058578 |
| [6] |
KAMEL A S, ABDELKADER N F, ABD EL-RAHMAN S S, et al. Stimulation of ACE2/ANG(1-7)/mas axis by diminazene ameliorates Alzheimer's disease in the D-galactose-ovariectomized rat model:Role of PI3K/Akt pathway[J]. Mol Neurobiol, 2018, 55(10): 8188-8202. DOI:10.1007/s12035-018-0966-3 |
| [7] |
QI Y F, ZHANG J, COLE-JEFFREY C T, et al. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology[J]. Hypertension, 2013, 62(4): 746-752. DOI:10.1161/HYPERTENSIONAHA.113.01337 |
| [8] |
BADAE N M, EL NAGGAR A S, EL SAYED S M, et al. Is the cardioprotective effect of the ACE2 activator diminazene aceturate more potent than the ACE inhibitor enalapril on acute myocardial infarction in rats?[J]. Can J Physiol Pharmacol, 2019, 97(7): 638-646. DOI:10.1139/cjpp-2019-0078 |
| [9] |
CHEN J J, CUI L Q, YUAN J L, et al. Protective effect of diminazene attenuates myocardial infarction in rats via increased inflammation and ACE2 activity[J]. Mol Med Rep, 2017, 16(4): 4791-4796. |
| [10] |
RAJAPAKSHA I G, MAK K Y, HUANG P, et al. The small molecule drug diminazene aceturate inhibits liver injury and biliary fibrosis in mice[J]. Sci Rep, 2018, 8(1): 10175. |
| [11] |
LI S M, WANG X Y, LIU F, et al. ACE2 agonist DIZE alleviates lung injury induced by limb ischemia-reperfusion in mice[J]. Sheng Li Xue Bao, 2018, 70(2): 175-183. |
| [12] |
MALEK M, NEMATBAKHSH M. The preventive effects of diminazene aceturate in renal ischemia/reperfusion injury in male and female rats[J]. Adv Prev Med, 2014, 2014: 740647. |
| [13] |
FRAGA-SILVA R A, COSTA-FRAGA F P, MONTECUCCO F, et al. Diminazene protects corpus cavernosum against hypercholesterolemia-induced injury[J]. J Sex Med, 2015, 12(2): 289-302. |
| [14] |
MALEK V, SHARMA N, SANKRITYAYAN H, et al. Concurrent neprilysin inhibition and renin-angiotensin system modulations prevented diabetic nephropathy[J]. Life Sci, 2019, 221: 159-167. DOI:10.1016/j.lfs.2019.02.027 |
| [15] |
GORU S K, KADAKOL A, MALEK V, et al. Diminazene aceturate prevents nephropathy by increasing glomerular ACE2 and AT2 receptor expression in a rat model of type1 diabetes[J]. Br J Pharmacol, 2017, 174(18): 3118-3130. DOI:10.1111/bph.13946 |
| [16] |
KANGUSSU L M, DE ALMEIDA T C S, PRESTES T R R, et al. Beneficial effects of the angiotensin-converting enzyme 2 activator dize in renovascular hypertension[J]. Protein Pept Lett, 2019, 26(7): 527-531. |
| [17] |
TAO L, QIU Y, FU X, et al. Angiotensin-converting enzyme 2 activator diminazene aceturate prevents lipopolysaccharide-induced inflammation by inhibiting MAPK and NF-κB pathways in human retinal pigment epithelium[J]. J Neuroinflammat, 2016, 13: 35. DOI:10.1186/s12974-016-0489-7 |
| [18] |
CAO X, YANG F Y, SHI T T, et al. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis[J]. Sci Rep, 2016, 6: 21592. DOI:10.1038/srep21592 |
| [19] |
BRUCE E B, SAKARYA Y, KIRICHENKO N, et al. ACE2 activator diminazene aceturate reduces adiposity but preserves lean mass in young and old rats[J]. Exp Gerontol, 2018, 111: 133-140. DOI:10.1016/j.exger.2018.07.008 |
| [20] |
RAFFAI G, KHANG G, VANHOUTTE P M. Angiotensin-(1-7) augments endothelium-dependent relaxations of porcine coronary arteries to bradykinin by inhibiting angiotensin-converting enzyme 1[J]. J Cardiovasc Pharmacol, 2014, 63(5): 453-460. DOI:10.1097/FJC.0000000000000069 |
| [21] |
YANG X H, WANG Y H, WANG J J, et al. Role of angiotensin-converting enzyme (ACE and ACE2) imbalance on tourniquet-induced remote kidney injury in a mouse hindlimb ischemia-reperfusion model[J]. Peptides, 2012, 36(1): 60-70. DOI:10.1016/j.peptides.2012.04.024 |
 2020, Vol. 46
2020, Vol. 46


