扩展功能
文章信息
- 历程, 刘慧, 郭亮, 马云飞, 刘百龙, 董丽华
- LI Cheng, LIU Hui, GUO Liang, MA Yunfei, LIU Bailong, DONG Lihua
- 放疗联合吉非替尼治疗EGFR敏感突变低级别肺黏液表皮样癌1例报告及文献复习
- Radiotherapy combined with gefitinib in treatment of low-grade pulmonary mucoepidermoid carcinoma with EGFR sensitive mutation: A case report and literature review
- 吉林大学学报(医学版), 2019, 45(06): 1436-1439
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1436-1439
- 10.13481/j.1671-587x.20190641
-
文章历史
- 收稿日期: 2019-04-01
2. 吉林大学第一医院病理科, 吉林 长春 130021
2. Department of Pathology, First Hospital, Jilin University, Changchun 130021, China
肺黏液表皮样癌(pulmonary mucoepidermoid carcinoma,PMEC)是一种罕见的肺恶性肿瘤,于1952年由Smetana首次提出,根据其组织分化程度可分为低级别与高级别两种[1]。低级别PMEC恶性度较高级别低,目前其主要的治疗方式为手术切除,大部分低级别PMEC患者可经手术切除根治。但对于不可切除的低级别PMEC,由于病例数较少,尚无标准的治疗模式。HAN等[1]报道PMEC患者表皮生长因子受体(epidermal growth factor receptor, EGFR)基因阳性突变率较高。国外已有EGFR-酪氨酸激酶抑制剂(tyrosine kinase inhibitors, TKIs)治疗PMEC患者的个案报道,但放疗与EGFR-TKIs的联合应用在国内外尚未见相关报道。本研究报道1例无法手术切除、基因检测结果显示为EGFR基因的21号外显子敏感突变的低级别PMEC患者,采用局部放疗联合吉非替尼的治疗模式,疗效较好,无进展生存期(progression free survival,PFS)至本研究截止已达18个月,本文作者结合相关文献复习探讨该治疗模式的有效性及安全性,以期为该病的治疗提供临床指导和参考。
1 临床资料 1.1 一般资料患者,女性,22岁,2017年7月因胸闷气短、干咳2个月入院。肺部CT显示:右肺门团块状高密度影,最大层面约7.7 cm× 7.0 cm,邻近右主支气管和右肺中间段支气管变窄,局部向支气管腔内突出。支气管镜下见右肺下叶开口新生物,堵塞管腔,活检病理:考虑为低级别PMEC。免疫组织化学:CK(+)、CgA (弱+)、Syn(—)、CD56(+)、EMA(+)、TTF-1(—)、Ki67(3%+)、Vimentin (—)、P40(+)、NapsinA(—)、CD117(+)、CK5/6(+)和P63(+)(图 1,见插页七)。半个月后,于北京煤炭总医院行气管镜下肿物部分切除术,术后病理显示:低级别PMEC。术后1周,患者出现咳血,每日咳血量50 ~ 100 mL,给予止血对症治疗后症状略减轻,为进一步治疗转入本院。相关检查:头部MRI和腹部CT未见转移灶,骨扫描未见放射性核素浓聚,临床分期为cT4N0M0,ⅢA期。
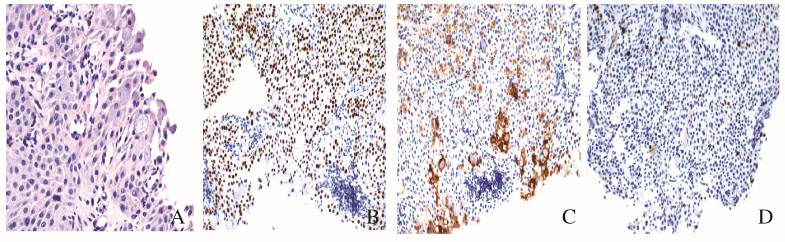
|
| A:Cells containing intracellular mucous (×400);B:Positive expression of P40 in squamous and intermediate cells (×200);C:Strongly positive expression of CD117 in mucous cells and weakly positive expression of CD117 in intermediate cells (×200);D:Low expression of Ki67 (×200). 图 1 HE染色检测PMEC患者的细胞形态表现 Fig. 1 Morphology of cells in PMEC patient detected by HE staining |
|
|
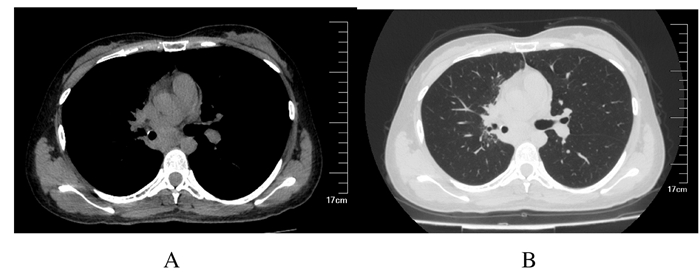
因肿瘤较大,堵塞气道,且存在咳血,决定给予患者右肺肿瘤放射治疗。勾画右肺肺门肿物为靶区体积(gross tumor volume, GTV),各方向外扩5 mm为肿瘤计划靶区(planned gross tumor volume,PGTV)。放疗1周时,基因检测结果回报:EGFR基因21号外显子L858R突变,组织丰度为1.7%。总剂量为12 Gy/6 f,开始同步口服吉非替尼250 mg·d-1。胸部放疗靶区剂量为50 Gy/25 f,复查肺CT显示:右肺门旁见团块状高密度影,右肺下叶支气管腔明显通畅,肿物缩小(图 2),咳血消失。

|
| A: Before radiotherapy; B: After radiotherapy; C:3 months after radiotherapy; D: 12 months after radiotherapy. 图 2 放疗前后PMEC患者胸部CT表现 Fig. 2 Thoracic CT images of PMEC patient before and after radiotherapy |
|
|
患者持续口服吉非替尼,250 mg·d-1,共17个月,定期复查肺CT,右肺肺门肿物缩小至3.6cm×2.8 cm(图 3),PFS已达18个月。病程中患者除轻度皮疹外,无其他不良反应,目前仍在随访中。
|
| A: Mediastinal window; B: Lung window. 图 3 放疗后18个月PMEC患者胸部CT表现 Fig. 3 Thoracic CT images of PMEC patient 18 months after radiotherapy |
|
|
PMEC是一种罕见的肺恶性肿瘤,于1952年由Smetana首次提出,在所有肺部肿瘤中仅占0.1% ~ 0.2%[1];PMEC起源于气管支气管树的小涎腺,通常发生在主支气管、肺叶支气管或段支气管。大多数患者表现为大气道刺激或阻塞的症状,包括咳嗽、咯血、喘息、胸痛和肺不张等。根据组织分化程度,PMEC可分为低级别和高级别两种[1-2]。PMEC的免疫组织化学检测结果中,CK7、Muc5Ac、P40和P63通常为阳性,而TTF-1、Calponin、HER2及ALK通常为阴性[3]。低级别PMEC患者较少发生淋巴结转移(低于5%),而高级别PMEC患者淋巴结转移率较高,侵袭性更强,具有更高的复发、转移风险,总生存期(overall survival,OS)与PFS明显低于低级别PMEC患者,预后较差。此外,年龄也是PMEC患者OS及PFS的影响因素[1, 4]。
PMEC目前主要的治疗方式为手术。研究[3, 5-6]显示:手术切除者超过90%,单纯手术后患者5年生存率高达87%。可手术切除的低级别PMEC经根治性手术可治愈[7-9]。对于局部晚期或发生转移等不可切除的PMEC患者,通常参照非小细胞肺癌(non small cell lung cancer, NSCLC)的治疗原则来治疗[1]。目前,对于不能手术的Ⅲ期NSCLC患者,放疗同步化疗仍是标准的治疗模式,可延长患者的OS [10]。研究[11]显示:放疗用于术后切缘阳性高级别PMEC患者的辅助治疗,但对患者的生存无影响。由于PMEC发病率较低,晚期患者的数量更少,目前尚无应用放疗、化疗和靶向药物治疗不可手术切除PMEC的相关研究,其疗效尚不明确。
HAN等[1]对5例PMEC患者进行了EGFR突变基因检测,显示其突变阳性率为60%(3/5),突变位点皆为L858R,HER2突变均为阴性。该患者病理类型为低级别PMEC,基因检测结果为EGFR基因突变阳性,突变位点为L858R第21外显子,与HAN等[1]的报道相符。由于EGFR突变率较高,建议PMEC患者行基因检测,如有突变可选择EGFR-TKIs治疗。EGFR-TKIs目前仍是EGFR突变阳性的晚期NSCLC患者一线治疗的药物,其中吉非替尼或厄洛替尼的治疗有效率高达70% ~ 80% [12]。
该患者肿瘤巨大,包绕肺门,无法行手术治疗,治疗前有咳血,病情凶险,且拒绝化疗,因此针对该患者的治疗,采取了局部放疗联合吉非替尼的治疗模式。研究[13-14]表明:辐射可导致EGFR表达增加,激活其下游的信号通路, 诱导细胞对放疗抵抗;而EGFR-TKIs在诱导细胞周期阻滞、细胞凋亡和DNA双链损伤修复等多个方面具有放射增敏作用。EGFR-TKIs与放疗联合应用可增加肿瘤细胞对放射治疗的敏感性。对于胸部放疗与EGFR-TKIs在NSCLC的联合应用中,国内外大部分研究纳入的患者其EGFR基因既有突变型也有野生型。XIA等[15]研究显示:联合应用治疗NSCLC的局控率可达96%,2年PFS为42%,OS为45%;而对于EGFR突变阳性患者,联合应用的疗效目前还缺乏相关Ⅱ期临床试验来证实。OKAMOT等[16]和MOSCHINI等[17]的研究表明:EGFR突变阳性的患者(2/3)局部控制较好,其PFS及OS均大于5年;WANG等[18]研究表明:对于EGFR突变阳性的Ⅲ/Ⅳ期NSCLC患者,局部放疗联合EGFR-TKIs局控率较好(约为84%),可明显延缓肿瘤的进展,提高患者的PFS及OS。在与治疗相关的不良反应中,最常见为皮疹,其余包括腹泻、厌食、疲劳和血液学毒性等。研究[17-19]表明:放疗与EGFR-TKIs联合应用可增加放射性肺炎的风险(1 ~ 2级常见,偶有3级),其具体机制还尚未明确,可能与放疗及EGFR-TKIs两者均可造成肺间质损伤有关,但总体的不良反应可以接受。
针对该患者的GIV,由于低级别PMEC患者较少发生淋巴结转移,且该患者CT检查未见阳性淋巴结,所以放射时将原发病灶勾画为GTV,外放5 mm为PGTV,不对淋巴引流区进行预防性照射。该患者肿瘤较大,同步口服吉非替尼可增加发生放射性肺炎的风险,因此选择了50 Gy/25 f的分割方式。放疗剂量为12 Gy/6 f时,患者咯血症状消失,疗效明显。经过胸部放疗同步口服吉非替尼治疗后,肿物缩小。患者持续口服吉非替尼至今,复查肺部CT显示肿物进一步缩小,无复发及远处转移,无放射性肺炎发生,疗效评价为部分缓解(partial remission, PR),PFS已达18个月,疗效满意,提高了患者的生活质量,该患者目前仍在随访中。
PMEC是一种罕见的肺恶性肿瘤,尚无标准的治疗模式。目前,尚无放疗联合EGFR-TKIs治疗不可切除、EGFR突变阳性低级别PMEC患者的相关报道。该患者经放疗联合吉非替尼治疗后取得了满意的疗效,且安全性高。对于EGFR突变阳性、不可切除的低级别PMEC患者,放疗联合EGFR-TKIs可以作为一种新的治疗模式。
| [1] |
HAN S W, KIM H P, JEON Y K, et al. Mucoepidermoid carcinoma of lung: potential target of EGFR-directed treatment[J]. Lung Cancer, 2008, 61(1): 30-34. |
| [2] |
YOUSEM S A, HOCHHOLZER L. Mucoepidermoid tumors of the lung[J]. Cancer, 1987, 60(6): 1346-1352. DOI:10.1002/1097-0142(19870915)60:6<1346::AID-CNCR2820600631>3.0.CO;2-0 |
| [3] |
HUO Z, WU H, LI J, et al. Primary pulmonary mucoepidermoid carcinoma: histopathological and moleculargenetic studies of 26 cases[J]. PLoS One, 2015, 10(11): e0143169. DOI:10.1371/journal.pone.0143169 |
| [4] |
ALSIDAWI S, MORRIS J C, WIKENHEISER-BROKAMP K A, et al. Mucoepidermoid carcinoma of the lung: a case report and literature review[J]. Case Rep Oncol Med, 2013, 2013: 625243. |
| [5] |
MOLINA J R, AUBRY M C, LEWIS J E, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors[J]. Cancer, 2007, 110(10): 2253-2259. DOI:10.1002/cncr.23048 |
| [6] |
XI JJ, JIANG W, LU S H, et al. Primary pulmonary mucoepidermoid carcinoma: an analysis of 21 cases[J]. World J Surg Oncol, 2012, 1(10): 232. |
| [7] |
OMESH T, GUPTA R, SAQI A, et al. A rare case of endobronchial mucoepidermoid carcinoma of the lung presenting as non-resolving pneumonia[J]. Respir MedCaseRep, 2018, 25: 154-157. |
| [8] |
SMETANA H F, IVERSON L, SWAN L L. Bronchogenic carcinoma; an analysis of 100 autopsy cases[J]. Mil Surg, 1952, 111(5): 335-351. |
| [9] |
VASASZ P, EGERVRY M. Mucoepidermoid bronchial tumors: a review of 34 operated cases[J]. Eur J Cardiothorac Surg, 2000, 17(5): 566-569. DOI:10.1016/S1010-7940(00)00386-9 |
| [10] |
CURRAN W J, PAULUS R. Sequential vs. concurrent chemoradiation for stage Ⅲ non-small cell lung cancer: randomized phase Ⅲ trial RTOG 9410[J]. J NatlCancerInst, 2011, 103(19): 1452-1460. |
| [11] |
EL M F, BEN S I, ISMALL O, et al. Mucoepidermoid carcinoma of the lung. A series of 10 cases[J]. Rev Pneumol Clin, 2005, 61(2): 78-82. DOI:10.1016/S0761-8417(05)84793-5 |
| [12] |
MITSUDOMI T, MORITA S, YATABE Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial[J]. Lancet Oncol, 2010, 11(2): 121-128. DOI:10.1016/S1470-2045(09)70364-X |
| [13] |
CHINNAIYAN P, HUANG S, VALLABHANENI G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva)[J]. Cancer Res, 2005, 65(8): 3328-3335. DOI:10.1158/0008-5472.CAN-04-3547 |
| [14] |
JIANG T, MIN W, LI Y, et al. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta-analysis[J]. Cancer Med, 2016, 5(6): 1055-1065. DOI:10.1002/cam4.673 |
| [15] |
WANG J, XIA T Y, WANG Y J, et al. Prospective study of epidermal growth factor receptor tyrosine kinase inhibitors concurrent with individualized radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer[J]. Int J Radiat Oncol Biol Phys, 2011, 81(3): e59-65. DOI:10.1016/j.ijrobp.2010.12.035 |
| [16] |
OKAAMOTO I, TAKAHASHI T, OKAMOTO H, et al. Single-agent gefitinib with concurrent radiotherapy for locally advanced non-small cell lung cancer harboring mutations of the epidermal growth factor receptor[J]. Lung Cancer, 2011, 72(2): 199-204. |
| [17] |
MOSCHINI I, DELL A C, LOSARDO P L, et al. Radiotherapy of non-small-cell lung cancer in the era of EGFR gene mutations and EGF receptor tyrosine kinase inhibitors[J]. Future Oncol, 2015, 11(16): 2329-2342. DOI:10.2217/fon.15.156 |
| [18] |
WANG Y, LI Y, XIA L, et al. Continued EGFR-TKI with concurrent radiotherapy to improve time to progression (TTP) in patients with locally progressive non-small cell lung cancer (NSCLC) after front-line EGFR-TKI treatment[J]. Clin Transl Oncol, 2018, 20(3): 366-373. DOI:10.1007/s12094-017-1723-1 |
| [19] |
ZHUANG H, YUAN Z, CHANG J Y, et al. Radiation pneumonitis in patients with non-small-cell lung cancer treated with erlotinib concurrent with thoracic radiotherapy[J]. J Thorac Oncol, 2014, 9(6): 882-885. DOI:10.1097/JTO.0000000000000126 |
 2019, Vol. 45
2019, Vol. 45


