扩展功能
文章信息
- 李文涛, 刘长红, 周晓华, 安力彬
- LI Wentao, LIU Changhong, ZHOU Xiaohua, AN Libin
- 下丘脑-垂体-肾上腺轴紊乱对脊髓损伤大鼠抑郁样行为的影响及其机制
- Effect of hypothalamic-pituitary-adrenal axis disorder on depression-like behavior in rats with spinal cord injury and its mechanism
- 吉林大学学报(医学版), 2019, 45(06): 1395-1400
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1395-1400
- 10.13481/j.1671-587x.20190634
-
文章历史
- 收稿日期: 2019-10-05
2. 黑龙江中医药大学佳木斯学院临床护理教研室, 黑龙江 佳木斯 154007
2. Department of Clincal Nursing, Jiamusi College, Heilongjiang University of Chinese Medicine, Jiamusi 154007, China
流行病学研究[1-2]显示:脊髓损伤(spinal cord injury,SCI)患者重度抑郁症发病率约为30%,是一般人群的2~3倍。国内外研究[3-4]表明下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal,HPA)轴紊乱与抑郁症的发生发展有密切关系。SCI后HPA轴立即激活,糖皮质激素水平随之升高[5],从而导致HPA轴功能紊乱,但引起SCI患者抑郁的具体作用机制尚不明确。本研究探讨SCI模型大鼠HPA轴功能紊乱对海马组织中Caspase-3蛋白表达的影响和SCI后抑郁的可能发病机制。
1 材料与方法 1.1 实验动物、主要试剂和仪器清洁雄性SD大鼠40只,体质量(260±20)g,由大连医科大学实验动物中心提供,动物合格证号:SCXK(辽)2018-003。PVDF膜购自美国Millipore公司,兔抗大鼠一抗Caspase-3、兔抗大鼠一抗β-actin和山羊抗兔HRP-二抗均购自美国Abcam公司,ECL增强化学发光试剂盒和BCA试剂盒定量购自上海碧云天生物技术有限公司,皮质酮(corticosterone, CORT)和促肾上腺皮质激素(adrenocorticotropic hormone, ACTH) ELISA试剂盒购自南京建成生物工程研究所。ChemiDocTM XRS+Imager凝胶成像系统购自美国Bio-Rad公司,Supermaze动物行为分析软件购自上海欣软信息科技有限公司。自制打击器。
1.2 实验分组和模型制备40只大鼠在实验室自制打击器中常规单笼饲养适应1周后,按照随机数字表法分为3组,分别为正常组12只、假模组12只和SCI组16只。如文献[6]所述,通过腹膜内注射10%水合氯醛麻醉28只假模组和SCI组大鼠。将16只SCI组大鼠以仰卧位置放在手术台上,触诊肋骨以定位T10胸椎的棘突。在背部手术区剃掉毛发并以碘伏消毒,然后用精细的手术刀做15mm中线切口,露出T10椎骨的背部,并用骨夹去除T10椎板的棘突和背部,直到脊髓硬膜外暴露。从10 mm的距离下落25 g打击棒直接击中暴露的脊髓,导致脊髓挫伤。成功挫伤的指标包括局部脊髓发红和肿胀、撞击后立即摇动两肢和大鼠双侧后肢麻痹,然后以手术缝合线将周围组织分层缝合。假模组12只大鼠在T10处只打开椎板,暴露脊髓,不进行打击。手术后将大鼠立即置于温水垫上直至恢复意识。所有大鼠术后连续3 d腹膜内注射青霉素(160 mg·kg-1),每天1次,连续3 d,以预防感染。手术次日开始每天按摩膀胱2次,直到大鼠恢复自动排尿。观察手术切口有无异常、腹部皮肤有无压疮或感染、下肢有无溃烂、排便有无异常等,注意鼠笼清洁卫生,及时更换垫料。抓取大鼠注意动作轻柔,避免牵拉损伤的脊髓。
1.3 旷场实验选择安静的房间,并设定实验时间为9:00—14:00。实验装置由100 cm(长)×100 cm(宽)×20 cm(高)的正方形木箱构成。将大鼠置于箱子中心位置,采用Supermaze软件录制和记录大鼠5 min内在木箱内自主活动总路程。消毒箱子后,再进行下一只大鼠的实验观察[7]。
1.4 蔗糖偏好实验选择安静的房间,将1个装有约250 mL的2%蔗糖溶液瓶和1个装有等量纯净水的溶液瓶放置在测试大鼠笼子的两侧,1 h后颠倒2个溶液瓶的位置,再放置1 h,防止位置偏差混淆结果。2 h后实验结束,测定每瓶溶液的质量变化[7]。然后使用以下公式计算蔗糖偏好百分比:蔗糖偏好百分比=蔗糖溶液摄入量(mL)/[蔗糖溶液摄入量(mL)+水摄入量(mL)]×100%。
1.5 强迫游泳实验在直径15 cm、高50 cm的透明玻璃筒中盛入30 cm深的清水,水温22℃ ~25℃。将大鼠放入水中,观察、记录大鼠在10 min内的不动时间[7]。
1.6 动物取材和指标检测大鼠在强迫游泳结束后2 d采用10%水合氯醛腹腔麻醉,断头取血,然后迅速在冰盒上取出海马组织放入液氮中快速冷冻,-80℃冻存,用于海马组织中Caspase-3蛋白表达水平的检测。血液室温静止0.5 h后,使用离心机2 500 r·min-1离心20 min,取出血清转移至新EP管中,置于-80℃冻存,采用ELISA试剂盒测定大鼠血清ACTH和CORT水平。采用组织蛋白裂解液提取大鼠海马组织中蛋白,然后用BCA试剂盒定量。SDS-PAGE凝胶上电泳,转移到PVDF膜,在5%脱脂牛奶中室温下封闭1 h,敷一抗兔抗大鼠Caspase-3(1:300)、兔抗大鼠β-actin(1:1 000),4℃过夜。TBST洗涤3次,每次10 min,然后将PVDF膜与山羊抗兔HRP-二抗在室温下孵育1 h;在TBST中洗涤3次,每次10 min,最后通过ECL增强化学发试剂盒在ChemiDocTM XRS+Imager成像系统上显影,通过ImageJ软件测量所有蛋白质的灰度值,计算目的蛋白表达水平。目的蛋白表达水平=目的蛋白质灰度值/内参β-actin灰度值。
1.7 统计学分析采用SPSS 24.0统计软件和GraphPad Prism 5.0进行统计学分析和图表绘制。各组大鼠旷场实验自主活动总路程、蔗糖偏好百分比、强迫游泳不动时间、血清CORT和ACTH水平以及海马组织中Caspase-3蛋白表达水平以x±s表示,旷场实验自主活动总路程、蔗糖偏好百分比、强迫游泳不动时间、血清CORT和ACTH水平多组间比较采用重复测量方差分析(Two-way ANOVA),海马组织中Caspase-3蛋白表达水平多组间比较采用单因素方差分析(One-way ANOVA),组间两两比较方差齐时采用LSD检验,方差不齐采用Dunnett’ s T3检验。以P<0.05为差异有统计学意义。
2 结果 2.1 各组大鼠行为学测试指标各组大鼠旷场实验结果表明:不同时间点各组大鼠在旷场实验中自主活动总路程比较差异有统计学意义(F=23.950,P=0.000)。见表 1。
| (n=30, x±s, l/mm) | |||||||||||||||||||||||||||||
| Group | Total distance | ||||||||||||||||||||||||||||
| (t/d) 0 | 9 | 33 | |||||||||||||||||||||||||||
| Normal | 20 382.28±1 829.60 | 18 010.73±2 207.10* | 16 026.51±1 775.59* | ||||||||||||||||||||||||||
| Sham model | 20 507.81±1 829.59 | 18 435.90±2 207.13* | 14 312.00±1 775.60* | ||||||||||||||||||||||||||
| SCI | 19 428.57±1 829.50 | 12 407.99±2 207.14*△ | 11 353.23±1 775.61*△ | ||||||||||||||||||||||||||
| * P < 0.01 vs 0 d; △ P < 0.01 vs normal group. | |||||||||||||||||||||||||||||
各组大鼠蔗糖偏好百分比检测结果表明:不同时间点蔗糖偏好百分比比较差异有统计学意义(F=11.99,P=0.001), 基线时间点各组大鼠蔗糖偏好百分比比较差异无统计学意义(P>0.05)。与基线时间点比较,损伤后第9和33天各组大鼠蔗糖偏好百分比差异有统计学意义(P < 0.01)。不同组别大鼠蔗糖偏好百分比比较差异有统计学意义(F=3.945,P=0.046),SCI组和正常组大鼠蔗糖偏好百分比比较差异有统计学意义(P < 0.05)。见表 2。
| (n=30, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | Percentage of sucrose preference | ||||||||||||||||||||||||||||
| (t/d) 0 | 9 | 33 | |||||||||||||||||||||||||||
| Normal | 68.82±3.92 | 87.60±4.57* | 93.23±6.74* | ||||||||||||||||||||||||||
| Sham model | 68.79±3.92 | 82.91±4.57* | 86.24±6.74* | ||||||||||||||||||||||||||
| SCI g | 67.34±3.58 | 70.26±4.17*△ | 80.90±6.16*△ | ||||||||||||||||||||||||||
| * P < 0.01 vs 0 d; △ P < 0.01 vs normal group. | |||||||||||||||||||||||||||||
各组大鼠强迫游泳不动时间观测结果显示:不同时间点各组大鼠强迫游泳不动时间比较差异有统计学意义(F=104.281,P=0.000),不同组别大鼠相同时间点不动时间比较差异有统计学意义(F=17.024,P=0.000),时间和组别之间存在交互作用(F=58.036,P=0.000)。在时间的单独效应方面,基线时间点各组大鼠强迫游泳不动时间比较差异无统计学意义(P>0.05),在损伤后第34天,与正常组和假模组比较,SCI组大鼠强迫游泳不动时间差异均有统计学意义(P < 0.01)。在组别的单独效应方面,正常组和假模组大鼠在基线时间点和损伤后第34天时强迫游泳不动时间比较差异均无统计学意义(P>0.05)。与基线时间点比较,SCI组大鼠损伤后第34天强迫游泳不动时间差异有统计学意义(P < 0.01)。见表 3。
| (n=30, x±s, t/s) | |||||||||||||||||||||||||||||
| Group | Immobility time | ||||||||||||||||||||||||||||
| (t/d) 0 | 34 | ||||||||||||||||||||||||||||
| Normal | 126.92±3.24 | 131.90±4.53* | |||||||||||||||||||||||||||
| Sham model | 127.21±3.25 | 134.05±4.51* | |||||||||||||||||||||||||||
| SCI | 125.38±3.24 | 183.35±4.53*△# | |||||||||||||||||||||||||||
| * P < 0.01 vs 0 d; △ P < 0.01 vs normal group; # P < 0.01 vs sham model group. | |||||||||||||||||||||||||||||
各组大鼠血清CORT水平检测结果表明:不同时间点血清CORT水平比较差异有统计学意义(F=22.869,P=0.000),不同组别间大鼠血清CORT水平比较差异有统计学意义(F=17.265,P=0.000),时间和组别之间存在交互作用(F=7.753, P=0.000)。在时间的单独效应方面,基线时间点各组大鼠血清CORT水平比较差异无统计学意义(P>0.05);在损伤后第9天,与正常组比较,假模组和SCI组大鼠血清CORT水平差异均有统计学意义(P < 0.01);在损伤后第36天,与正常组和假模组比较,SCI组大鼠血清CORT水平差异均有统计学意义(P < 0.01)。在组别的单独效应方面,正常组大鼠在基线时间点和损伤后第9天和损伤后第36天组间两两比较差异均无统计学意义(P>0.05)。假模组大鼠损伤后第9天和损伤后第36天与基线时间点血清CORT水平比较差异均有统计学意义(P < 0.05),损伤后第9天和第36天血清CORT水平比较差异有统计学意义(P < 0.05)。SCI组大鼠损伤后第9天和第36天与基线时间点比较差异均有统计学意义(P < 0.01),损伤后第9天和第36天比较差异无统计学意义(P>0.05)。各组大鼠血清ACTH水平检测结果表明:不同时间点各组大鼠血清ACTH水平比较差异有统计学意义(F=36.598,P=0.000),不同组别大鼠血清ACTH水平比较差异有统计学意义(F=55.314,P=0.000),时间和组别之间存在交互作用(F=6.452, P=0.000)。在时间的单独效应方面,基线时间点各组大鼠血清ACTH水平比较差异无统计学意义(P>0.05);在损伤后第9天和第36天,与正常组与假模组比较,SCI组大鼠血清ACTH水平差异均有统计学意义(P < 0.01)。在组别的单独效应方面,正常组在基线时间点、损伤后第9天和损伤后第36天血清ACTH水平比较差异均无统计学意义(P>0.05)。假模组大鼠损伤后第9天、基线时间点和损伤后第36天血清ACTH水平比较差异有统计学意义(P < 0.05)。SCI组大鼠损伤后第9天和第36天血清ACTH水平与基线时间点比较差异均有统计学意义(P < 0.01),损伤后第9和36天血清ACTH水平比较差异无统计学意义(P>0.05)。见表 4和5。
| [n=18, x±s, ρB/(μg·L-1)] | |||||||||||||||||||||||||||||
| Group | CORT | ||||||||||||||||||||||||||||
| (t/d) 0 | 9 | 36 | |||||||||||||||||||||||||||
| Normal | 128.50±7.24 | 135.15±11.40 | 148.18±9.44 | ||||||||||||||||||||||||||
| Sham model | 128.44±7.23 | 189.27±11.41*# | 159.28±9.45#○ | ||||||||||||||||||||||||||
| SCI | 131.06±7.24 | 210.20±11.40*△# | 227.58±9.44*△# | ||||||||||||||||||||||||||
| * P < 0.01 vs normal group; △ P < 0.01 vs sham model group; # P < 0.01 vs 0 d; ○ P < 0.01 vs 9 d. | |||||||||||||||||||||||||||||
| Group | ACTH | ||
| (t/d) 0 | 9 | 36 | |
| Normal | 64.02±2.57 | 62.62±6.51 | 61.66±6.30 |
| Sham model | 64.40±2.58 | 80.16±6.52# | 63.47±6.29○ |
| SCI | 65.64±2.57 | 150.56±6.51*△# | 156.25±6.31*△# |
| * P < 0.01 vs normal group; △ P < 0.01 vs sham model group; # P < 0.01 vs 0 d; ○ P < 0.01 vs 9 d. | |||
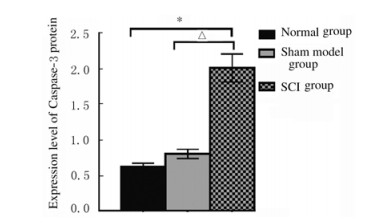
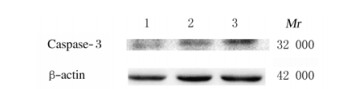
各组大鼠海马组织中Caspase-3蛋白表达水平比较差异有统计学意义(F=169.937,P=0.000)。与正常组和假模组比较,SCI组大鼠海马组织中Caspase-3蛋白表达水平升高(P < 0.01)。见图 1和2。
|
| * P < 0.01 vs normal group; △ P < 0.01 vs sham model group. 图 1 各组大鼠海马组织中Caspase-3蛋白表达水平 Fig. 1 Expression levels of Caspase-3 protein in hippocampus tissue of rats in various groups |
|
|
|
| Lane 1:Normal group; Lane 2:Sham model group; Lane 3:SCI group. 图 2 各组大鼠海马组织中Caspase-3蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of Caspase-3 protein in hippocampus tissue of rats in various groups |
|
|
慢性应激导致机体神经内分泌免疫网络失衡,糖皮质激素水平短期内升高可以帮助机体抵御外界刺激,然而其持续升高则对机体产生不利影响。海马是情绪相关的重要脑区,富含丰富的糖皮质激素受体(glucocorticoid receptor,GR),是应激损伤的主要靶器官。慢性应激时海马率先受到攻击,海马损伤进一步加重HPA轴功能紊乱,从而在抑郁病理进程中发挥重要作用[8-9]。目前公认的抑郁模型常采用的行为测试方法主要有蔗糖偏好实验、旷场实验和强迫游泳实验等,其分别代表抑郁的不同表现,均能对抑郁症状进行有效评估。蔗糖偏好实验是评估快感缺失的金标准[10-12];强迫游泳实验用来评估无助感,以绝望状态的持续时间作为抑郁症的评价指标[13-14];旷场实验评估大鼠在新环境中的动机和探索兴趣、焦虑行为和紧张程度[4]。本研究结果显示:SCI组大鼠出现抑郁样行为,表现在自主活动总距离减少和强迫游泳不动时间延长,这与多数研究[4, 15-16]结果一致,上述研究结果均表明SCI后大鼠会出现抑郁样的行为。
本研究结果显示:SCI大鼠血清CORT和ACTH水平高于正常组和假模组,但是CORT水平在SCI动物模型中变化的时间点存在争议。有学者[5]发现血清CORT水平在SCI后24 h明显增加,SCI后14 d下降,但SCI后28 d时血清CORT水平高于14 d,低于手术后的第1天。另有学者研究[17]结果显示:SCI后从SCI开始直到第28天,大鼠血清CORT水平呈逐渐升高的趋势,而假模组大鼠血清CORT水平逐渐降低。尽管不同文献中关于血清CORT水平的报道不一致,但都得出一个共同的结论,即SCI后HPA轴活性异常,从而可能导致抑郁行为的发生。
Caspase家族在抑郁发病中可能起重要作用,其中Caspase-3是介导细胞凋亡的核心蛋白酶[18]。慢性应激抑郁大鼠海马Caspase-3蛋白表达水平升高,海马神经元凋亡[19-20]。本研究也有相同的研究结论,其可能是SCI后的慢性应激引起HPA轴紊乱造成海马细胞凋亡增强和海马区脑组织损伤。
综上所述,SCI大鼠HPA轴功能亢进,致使海马Caspase-3蛋白表达增加,海马功能受损出现抑郁样行为,从而降低HPA轴的负反馈调节功能,造成恶性循环,促进疾病的发生发展。后续将进一步研究SCI后抑郁的深层发病机制,以期为其防治提供依据。
| [1] |
KESSLER R C, PETUKHOVA M, SAMPSON N A, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States[J]. Int J Methods Psychiatr Res, 2012, 21: 169-184. DOI:10.1002/mpr.1359 |
| [2] |
KRUEGER H, NOONAN V K, WILLIAMS D, et al. The influence of depression on physical complications in spinal cord injury: behavioral mechanisms and health-care implications[J]. Spinal Cord, 2013, 51: 260-266. DOI:10.1038/sc.2013.3 |
| [3] |
LI JG, CHEN J, MA N, et al. Effects of corticosterone on the expression of mature brain-derived neurotrophic factor (mBDNF) and proBDNF in the hippocampal dentate gyrus[J]. Behav Brain Res, 2019, 365: 150-156. DOI:10.1016/j.bbr.2019.03.010 |
| [4] |
QIU Z K, ZHANG G H, ZHONG D S, et al. Puerarin ameliorated the behavioral deficits induced by chronic stress in rats[J]. Sci Rep, 2017, 7(1): 6266. |
| [5] |
POPOVICH P G, STUCKMAN S, GIENAPP I E. Alterations in immune cell phenotype and function after experimental spinal cord injury[J]. J Neurotrauma, 2001, 18(9): 957-966. DOI:10.1089/089771501750451866 |
| [6] |
ZHAO B L, LI W T, ZHOU X H, et al. Effective robotic assistive pattern of treadmill training for spinal cord injury in a rat model[J]. Exp Ther Med, 2018, 15(4): 3283-3294. |
| [7] |
LUEDTKE K, BOUCHARD S M, WOLLER S A, et al. Assessment of depression in a rodent model of spinal cord injury[J]. J Neurotrauma, 2014, 31(12): 1107-1121. DOI:10.1089/neu.2013.3204 |
| [8] |
DAODEE S, MONTHAKANTIRAT O, RUENGWINITWONG K, et al. Effects of the ethanol extract of dipterocarpus alatus leaf on the unpredictable chronic mild stress-induced depression in icr mice and its possible mechanism of action[J]. Molecules, 2019, 24(18): E3396. DOI:10.3390/molecules24183396 |
| [9] |
SOLOMON M B, LOFTSPRING M, DE KLOET A D, et al. Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice[J]. Endocrinology, 2015, 156(8): 2843-2853. DOI:10.1210/en.2015-1276 |
| [10] |
LIANG X, TANG J, CHAO F L, et al. Exercise improves depressive symptoms by increasing the number of excitatory synapses in the hippocampus of CUS-Induced depression model rats[J]. Behav Brain Res, 2019, 374: 112115. DOI:10.1016/j.bbr.2019.112115 |
| [11] |
ZHANG Y, HUANG R R, CHENG M J, et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2[J]. Microbiome, 2019, 7(1): 116. DOI:10.1186/s40168-019-0733-3 |
| [12] |
TANG J, LIANG X, ZHANG Y, et al. The effects of running exercise on oligodendrocytes in the hippocampus of rats with depression induced by chronic unpredictable stress[J]. Brain Res Bull, 2019, 149: 1-10. DOI:10.1016/j.brainresbull.2019.04.001 |
| [13] |
YANKELEVITCH-YAHAV R, FRANKO M, HULY A, et al. The forced swim test as a model of depressive-like behavior[J]. J Vis Exp, 2015(97): 52587. |
| [14] |
TUNG T H, TUNG Y T, LIN I H, et al. Fish oil, but not olive oil, ameliorates depressive-like behavior and gut microbiota dysbiosis in rats under chronic mild stress[J]. Biomolecules, 2019, 9(10): E516. DOI:10.3390/biom9100516 |
| [15] |
DO ESPÍRÍTO SANTO C C, DA SILVA FIORIN F, ILHA J, et al. Spinal cord injury by clip-compression induces anxiety and depression-like behaviours in female rats: The role of the inflammatory response[J]. Brain Behav Immun, 2019, 78: 91-104. DOI:10.1016/j.bbi.2019.01.012 |
| [16] |
MALDONADO-BOUCHARD S, PETERS K, WOLLER S A, et al. Inflammation is increased with anxiety- and depression-like signs in a rat model of spinal cord injury[J]. Brain Behav Immun, 2016, 51: 176-195. DOI:10.1016/j.bbi.2015.08.009 |
| [17] |
ZHANG Y, GUAN Z, READER B, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury[J]. J Neurosci, 2013, 33: 12970-12981. DOI:10.1523/JNEUROSCI.1974-13.2013 |
| [18] |
CHOUDHARY G S, AL-HARBI S, ALMASAN A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis[J]. Methods Mol Biol, 2015, 1219: 1-9. |
| [19] |
ARORA V, CHOPRA K. Possible involvement of oxido-nitrosative stress induced neuro-inflammatory cascade and monoaminergic pathway: underpinning the correlation between nociceptive and depressive behaviour in a rodent model[J]. J Affect Disord, 2013, 151: 1041-1052. DOI:10.1016/j.jad.2013.08.032 |
| [20] |
肖仕和, 刘仲海, 陈晓光. 舒肝颗粒对抑郁模型大鼠海马神经元凋亡、脑组织caspase-3蛋白及外周血中细胞因子水平的影响[J]. 中国地方病防治杂志, 2017, 32(5): 496, 498. |
 2019, Vol. 45
2019, Vol. 45


