扩展功能
文章信息
- 刘翼, 祝琳, 康敏
- LIU Yi, ZHU Lin, KANG Min
- miR-302b-3p对食管癌EC-109细胞增殖和凋亡的影响及其机制
- Effect of miR-302b-3p on proliferation and apoptosis of esophageal cancer EC-109 cells and its mechanism
- 吉林大学学报(医学版), 2019, 45(06): 1346-1352
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1346-1352
- 10.13481/j.1671-587x.20190626
-
文章历史
- 收稿日期: 2018-11-23
2. 西南医科大学附属医院耳鼻咽喉头颈外科, 四川 泸州 646000
2. Department of Otolaryngology and Head-Neck Surgery, Affiliated Hospital, Southwest Medical University, Luzhou 646000, China
微小RNAs(miRNAs)是一类由18~25 nt组成的非编码RNA,可通过直接与靶基因的3′端非翻译区(3′-UTR)相互作用调控靶基因的表达,参与细胞的增殖、分化和凋亡等生物过程,与包括食管癌在内的多种疾病的发生发展有密切关联[1-2]。miR-302b-3p被证实在食管癌组织中表达下调,不仅可通过靶向ERBB4、Irf2和CXCR参与调控食管癌的炎症反应,还可通过靶向ERBB4诱导癌细胞凋亡和抑制细胞增殖[3-4];然而其调控食管癌细胞增殖和凋亡的分子机制尚未见报道。上皮细胞转化序列2(epithelial cell transformation sequence 2, ECT2)基因被证实是一种重要的致癌基因,在乳腺癌和肺腺癌等细胞增殖和凋亡过程中发挥重要作用[5-6]。本研究通过生物信息学软件预测发现ECT2 3′-UTR存在能够与miR-302b-3p互补结合的核苷酸序列,猜测ECT2可能是miR-302b-3p的潜在靶基因。本研究以人食管癌EC-109细胞作为研究对象,观察miR-302b-3p对食管癌EC-109细胞增殖和凋亡的影响及其对ECT2的靶向调控作用,旨在阐明miR-302b-3p调控食管癌细胞增殖和凋亡的分子机制是否与ECT2有关。
1 材料与方法 1.1 细胞、主要试剂和仪器人食管癌EC-109细胞和人正常食管上皮HET-1A细胞(美国ATCC公司)。胎牛血清(兰州民海生物工程有限公司),RPMI-1640培养基(美国Gibco公司),胰蛋白酶、二甲基亚枫、噻唑蓝(methylthiazolyldiphenyl-tetrazolium bromide,MTT)试剂(美国Sigma公司),miR-302b-3p mimics/inhibitor及其阴性对照片段(江苏百奥迈科生物技术公司),青链霉双抗混合液(美国Gemini公司),细胞周期蛋白D1(CyclinD1)抗体、细胞周期蛋白依赖性激酶2(recombinant cyclin dependent kinase 1, CDK2)抗体、ECT2抗体、Bcl-2相关X蛋白(Bcl-2 associated X protein, Bax)抗体、活化的含半胱氨酸的天冬氨酸蛋白水解酶3(Cleaved cysteinyl aspartate specific proteinase-3,Cleaved caspase-3)抗体、β肌动蛋白(β-actin)抗体(武汉博士德生物公司),硝酸纤维素膜(nitrocellulose filter membrane, NC)(美国Pierce公司),辣根过氧化酶标记的二抗(北京中杉金桥生物有限公司),报告基因表达载体(美国Ambion公司),二喹啉甲酸(bicinchoninic acid, BCA)蛋白浓度测定试剂盒、电化学发生(electrochemical luminescence,ECL)试剂盒(上海碧云天生物技术研究所),LipofectamineTM2000、实时荧光定量PCR试剂盒、cDNA合成试剂盒(美国Invitrogen公司),TRIzol总RNA抽提试剂盒(北京百泰克生物公司),双荧光素酶检测试剂盒(美国Promega公司),细胞凋亡检测试剂盒(美国BD公司)。流式细胞仪(美国BD公司),PCR扩增仪(美国MJ公司),UVP凝胶图象分析仪(英国Gene Genius公司),CO2细胞培养箱(上海博迅医疗仪器有限公司)。
1.2 细胞培养采用含有10%胎牛血清和双抗的RPMI-1640培养基于体积分数为5%CO2、温度为37℃、饱和湿度细胞培养箱中培养EC-109和HET-1A细胞。每隔2 d更换新鲜培养液。待细胞铺满瓶底时,以0.25%胰蛋白酶消化传代。收集生长良好的对数生长期细胞进行实验。
1.3 细胞分组和转染将EC-109细胞分为空白对照组(未转染)、miR-NC组(转染miR-302b-3p mimics阴性对照)、miR-302b-3p组(转染miR-302b-3p mimics)、anti-miR-NC组(转染miR-302b-3p inhibitor阴性对照)和anti-miR-302b-3p组(转染miR-302b-3p inhibitor), 每组设3个复孔。将EC-109细胞以每孔500 μL(约3×106个)接种至6孔细胞板上,培养箱内培养过夜。待细胞贴壁生长约为75%融合时,参照LipofectamineTM2000说明书步骤将配制的脂质体miRNA混合物按照实验分组加入到EC-109细胞中。转染6 h后更换含胎牛血清的新鲜培养基。继续培养48 h后,收集各组细胞进行后续实验。
1.4 实时荧光定量PCR法检测HET-1A细胞和EC-109细胞中miR-302b-3p表达水平收集未转染的HET-1A细胞、EC-109细胞和转染48 h的各组EC-109细胞,采用TRIzol法提取总RNA,采用分光光度计测定RNA的浓度和完整性。参照cDNA合成试剂盒说明书步骤反转录合成cDNA。以cDNA为模板进行PCR扩增。20 μL反应体系:2 μL cDNA,加入10 μL 2×定量PCR Master Mix、各0.08 μL上下游引物(摩尔浓度为20 μmol·L-1)、0.4 μLTaq DNA聚合酶(2.5 U·μL-1)和6 μL ddH2O。PCR反应体系:95 ℃预变性3 min,95 ℃变性30 s、60 ℃复性30 s,72℃延伸30 s,共40个循环。miR-302b-3p引物:F 5′-ATCCAGTGCGTGTCGTG-3′,R 5′-TGCTTAAGTGCTTCCATGTT-3′;U6引物:F 5′-ATTGGAACGATACAGAGAAGATT-3′,R 5′-GGAACGCTTCACGAATTTG -3′。以U6为内参,采用2-△△Ct法计算miR-302b-3p的相对表达水平。实验重复3次。
1.5 MTT法检测各组EC-109细胞活性以1 000 r·min-1离心5 min后收集各组EC-109细胞,调整细胞浓度为2×104 mL-1。以每孔100 μL平铺于96孔细胞板上,每组设3个平行孔。置于细胞培养箱内常规培养,分别在培养24、48和72 h后向每孔中加入10 μLMTT(5 g·L-1)溶液孵育4 h,并以100 μL二甲基亚砜溶解MTT结晶。采用酶标仪在450 nm波长处检测各组细胞的吸光度(A)值,重复测量3次,取A值平均值表示各组细胞活性。
1.6 流式细胞术检测各组EC-109细胞周期分布和细胞凋亡率收集各组EC-109细胞,采用预冷的磷酸缓冲液洗涤后,调整细胞密度为2×105个mL-1。分别参照细胞周期检测试剂盒和凋亡检测试剂盒说明书检测各组细胞的周期分布和细胞凋亡率。实验重复3次。其中细胞凋亡率的计算是将每次实验中得到的右上限和右下限结果之和为1次结果,实验结果以重复3次后的平均值表示。
1.7 Western blotting法检测各组细胞中CyclinD1、CDK2、Bax和Cleaved caspase-3蛋白表达水平向待测的EC-109细胞中加入细胞裂解液提取总蛋白后,采用BCA法检测总蛋白的浓度与纯度。按照每孔50 μg将蛋白样品上样至12%SDS-PAGE凝胶孔中,电泳至溴酚蓝跑出胶外,结束电泳。将分离的蛋白条带转至NC膜上。以5%去脂奶粉封闭2 h后,一抗于4 ℃下孵育24 h。封闭液洗涤3次(每次15 min)后,加入二抗于37 ℃孵育1 h。滴加ECL化学发光剂显影后,凝胶成像系统扫描分析。目的蛋白表达水平=目的蛋白条带灰度值/内参β-actin条带灰度值。结果以3次实验的均值表示。
1.8 双荧光素酶报告基因实验验证各组EC-109细胞的荧光素酶活性构建野生型ECT2-WT和突变型ECT2-MUT的ECT2 3′-UTR双荧光报告质粒。实验分为miR-NC/anti-miR-NC+ECT2-WT组、miR-302b-3p/anti-miR-302b-3p+ECT2-WT组、miR-NC/anti-miR-NC+ECT2-MUT组和miR-302b-3p/anti-miR-302b-3p+ECT2-MUT组。采用LipofectamineTM2000分别将miR-302-3p mimics、miR-302-3p inhibitor及其相应的阴性对照miR-NC、anti-miR-NC与ECT2-WT和ECT2-MUT质粒共转染至EC-109细胞中。转染24 h后,参照双荧光素酶报告基因检测试剂盒说明书检测各组EC-109细胞的相对荧光素酶活性,各组EC-109细胞的相对荧光素酶活性可以反映miR-302b-3p与ECT2的靶向关系。荧光素酶活性=萤火虫荧光强度/海肾荧光强度。实验重复3次。
1.9 统计学分析采用SPSS22.0统计软件进行统计学分析。各组细胞中miR-302b-3p的表达水平、细胞活性、周期分布和细胞凋亡率以及细胞中CyclinD1、CDK2、Bax、Cleaved caspase-3和ECT-2蛋白表达水平及EC-109细胞的荧光素酶活性以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用独立样本t检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组EC-109细胞中miR-302b-3p表达水平食管癌EC-109细胞中miR-302b-3p的表达水平(1.00±0.06)明显低于正常食管癌上皮HET-1A细胞(0.26±0.28)(t=4.476,P=0.011)。与空白对照组比较,miR-NC组和anti-miR-NC组EC-109细胞中miR-302b-3p的表达水平差异无统计学意义(P>0.05);miR-302b-3p组EC-109细胞中miR-302b-3p的表达水平较miR-NC组明显升高(P < 0.05),anti-miR-302b-3p组EC-109细胞中miR-302b-3p表达水平较anti-miR-NC组明显降低(P < 0.05)。见表 1。
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | miR-302b-3p | CyclinD1 protein | CDK2 protein | ||||||||||||||||||||||||||
| Blank control | 1.00±0.06 | 0.61±0.06 | 0.48±0.05 | ||||||||||||||||||||||||||
| miR-NC | 0.97±0.05 | 0.56±0.05 | 0.52±0.06 | ||||||||||||||||||||||||||
| miR-302b-3p | 2.56±0.32* | 0.19±0.02* | 0.23±0.03* | ||||||||||||||||||||||||||
| anti-miR-NC | 0.95±0.06 | 0.58±0.05 | 0.50±0.05 | ||||||||||||||||||||||||||
| anti-miR-302b-3p | 0.16±0.02△ | 0.85±0.06△ | 0.78±0.07△ | ||||||||||||||||||||||||||
| F | 102.023 | 66.631 | 39.604 | ||||||||||||||||||||||||||
| P | 0.000 | 0.000 | 0.000 | ||||||||||||||||||||||||||
| * P < 0.05 compared with miR-NC group; △ P < 0.05 compared with anti-miR-NC group. | |||||||||||||||||||||||||||||
与空白对照组比较,miR-NC组和anti-miR-NC组EC-109细胞活性和不同细胞周期EC-109细胞百分比比较差异无统计学意义(P>0.05);与miR-NC组比较,miR-302b-3p组EC-109细胞活性和S期EC-109细胞百分比明显降低(P < 0.05),G0/G1期EC-109细胞百分比升高(P < 0.05);与anti-miR-NC组比较,anti-miR-302b-3p组EC-109细胞活性和S期EC-109细胞百分比均明显升高(P < 0.05),G0/G1期EC-109细胞百分比明显降低(P < 0.05)。Western blotting法检测结果显示:miR-302b-3p组EC-109细胞中周期相关蛋白CyclinD1和CDK2的表达降低,CyclinD1和CDK2蛋白的表达升高。见图 1、表 1和2。

|
| Lane 1: Blank control group; Lane 2: miR-NC group; Lane 3: miR-302b-3pgroup; Lane 4: anti-miR-NC group; Lane 5: anti-miR-302b-3p group. 图 1 Western blotting法检测各组EC-109细胞中CyclinD1和CDK2蛋白表达电泳图 Fig. 1 Electrophoregram of expressions of CyclinD1 and CDK2 proteins in EC-109 cells in various groups detected by Western blotting method |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | Percentage of EC-109 cells (η/%) | Cell viability | |||||||||||||||||||||||||||
| G0/G1 | S | G2/M | 48 h | 72 h | |||||||||||||||||||||||||
| Blank control | 52.16±2.42 | 17.54±2.18 | 30.25±2.28 | 0.58±0.03 | 1.10±0.07 | ||||||||||||||||||||||||
| miR-NC | 51.64±2.85 | 16.35±1.95 | 32.02±1.96 | 0.61±0.03 | 1.12±0.06 | ||||||||||||||||||||||||
| miR-302b-3p | 65.23±3.24* | 9.62±1.22* | 31.79±2.15 | 0.40±0.03* | 0.67±0.05* | ||||||||||||||||||||||||
| anti-miR-NC | 52.25±3.06 | 18.05±2.23 | 32.68±1.85 | 0.56±0.04 | 1.08±0.06 | ||||||||||||||||||||||||
| anti-miR-302b-3p | 39.08±2.25△ | 25.47±2.02△ | 35.45±3.20 | 0.79±0.05△ | 1.34±0.10△ | ||||||||||||||||||||||||
| F | 32.982 | 24.978 | 1.992 | 42.728 | 35.988 | ||||||||||||||||||||||||
| P | 0.000 | 0.000 | 0.172 | 0.000 | 0.000 | ||||||||||||||||||||||||
| * P < 0.05 compared with miR-NC group; △P < 0.05 compared with anti-miR-NC group. | |||||||||||||||||||||||||||||
与miR-NC组比较,miR-302b-3p组EC-109细胞凋亡率和细胞中Bax及Cleaved caspase-3蛋白表达水平均明显升高(P < 0.05);与anti-miR-NC组比较,anti-miR-302b-3p组EC-109细胞凋亡率和细胞中Bax及Cleaved caspase-3蛋白表达水平均明显降低(P < 0.05);与空白对照组比较,miR-NC组和anti-miR-NC组EC-109细胞凋亡率及细胞中Bax和Cleaved caspase-3蛋白表达水平差异无统计学意义(P>0.05)。见图 2和3及表 3。
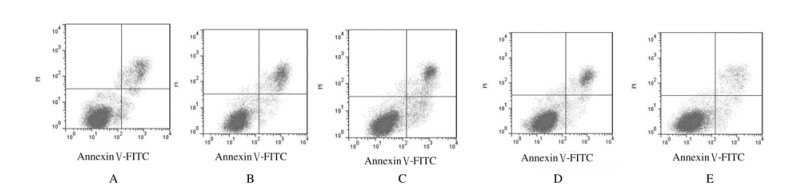
|
| A: Blank control group; B: miR-NC group; C: miR-302b-3p group; D: anti-miR-NC group; E: anti-miR-302b-3p group. 图 2 流式细胞术检测各组EC-109细胞的凋亡情况 Fig. 2 Apoptosis of EC-109 cells in various groups detected by flow cytometry |
|
|

|
| Lane 1: Blank control group; Lane 2: miR-NC group; Lane 3: miR-302b-3pgroup; Lane 4: anti-miR-NC group; Lane 5: anti-miR-302b-3p group. 图 3 Western blotting法检测各组EC-109细胞中Bax和Cleaved caspase-3蛋白表达电泳图 Fig. 3 Electrophoregram of expressions of Bax and Cleaved caspase-3 proteins in EC-109 cells in various groups detected by Western blotting method |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | Apoptotic rate(η/%) | Bax protein | Cleaved caspase-3 protein | ||||||||||||||||||||||||||
| Blank control | 7.85±1.02 | 0.38±0.03 | 0.32±0.02 | ||||||||||||||||||||||||||
| miR-NC | 8.26±0.93 | 0.41±0.03 | 0.29±0.03 | ||||||||||||||||||||||||||
| miR-302b-3p | 15.75±2.18* | 0.67±0.05* | 0.46±0.03* | ||||||||||||||||||||||||||
| anti-miR-NC | 6.57±0.86 | 0.37±0.03 | 0.28±0.03 | ||||||||||||||||||||||||||
| anti-miR-302b-3p | 2.16±0.22△ | 0.12±0.02△ | 0.08±0.01△ | ||||||||||||||||||||||||||
| F | 48.468 | 305.759 | 86.625 | ||||||||||||||||||||||||||
| P | 0.000 | 0.000 | 0.000 | ||||||||||||||||||||||||||
| * P < 0.05 compared with miR-NC group; △ P < 0.05 compared with anti-miR-NC group. | |||||||||||||||||||||||||||||
采用TargetScanHuman7.1生物信息学软件(http://www.targetscan.org/)预测发现ECT2 3′ UTR有与miR-302b-3p互补的核苷酸序列,见表 4。与miR-NC+ECT2-WT组(1.00±0.06)比较,miR-302b-3p+ECT2-WT组EC-109细胞的荧光素酶活性(0.42±0.03)降低(P < 0.05);与anti-miR-NC+ECT2-WT组(0.97±0.05)比较,anti-miR-302b-3p+ECT2-WT组EC-109细胞的荧光素酶活性(2.85±0.13)明显升高(P < 0.05)。与miR-NC+ECT2-MUT组(1.00±0.08)或anti-miR-NC+ECT2-MUT组(1.04±0.06)比较,miR-302b-3p+ECT2-MUT组(0.98± 0.05)和anti-miR-302b-3p+ECT2-MUT组(0.95±0.07)EC-109细胞荧光素酶活性差异无统计学意义(P>0.05)。Western blotting法检测结果显示:与miR-302b-3p过表达前(0.39±0.03)比较,miR-302b-3p过表达后EC-109细胞中ECT2蛋白表达水平(0.15±0.02)降低(P < 0.05);与miR-302b-3p低表达前(0.37±0.04)比较,miR-302b-3p低表达后EC-109细胞中ECT2蛋白表达水平(0.72±0.06)明显升高。见图 4。
| Gene | Complementary nucleotide sequence | Binding position |
| ECT2 | 5′-AUGGUACUUGUAAUUAG-CACUUG-3′ | 84-90 of ECT2 |
| miR-302b-3p | 3′-GAUGAUUUUGUACCUUCG-UGAAU-5′ | 3′ UTR |
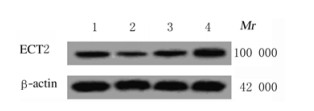
|
| Lane 1:miR-NC group; Lane 2:miR-302b-3p group; Lane 3:anti-miR-NC group; Lane 4:anti-miR-302b-3p group. 图 4 Western blotting法检测各组EC-109细胞ECT2蛋白表达电泳图 Fig. 4 Electrophoregram of expressions of ECT2 protein in EC-109cells in various groups detected by Western blotting method |
|
|
肿瘤发生发展过程中往往伴随着某些miRNAs的异常表达,而这些miRNAs在肿瘤细胞增殖、侵袭、转移和凋亡过程中发挥重要作用[7-8];其中,作为miR-302基因簇家族中的重要代表,miR-302b在多种肿瘤的进程中扮演着重要角色。miR-302b-3p在胃癌组织和细胞中表达水平下调,上调其表达可通过靶向胰岛素样生长因子1受体(insulin-like growth factor-1 receptor, IGF-1R)抑制AKT信号通路的激活,影响周期相关蛋白CyclinA2、CyclinD1、CDK2、CDK6和凋亡蛋白Bax、Bcl-2的表达发挥抑制细胞增殖、细胞周期G1→S转化并诱导细胞凋亡的作用[9]。同时,miR-302b还可通过靶向调控EphA2减弱Wnt/β-catenin/EMT信号级联通路抑制胃癌细胞的增殖、侵袭和迁移[10]。在恶性胸膜间皮瘤细胞中,miR-302b通过靶向Mcl-1抑制肿瘤细胞的增殖并诱导细胞凋亡[11]。肝癌组织中miR-302b表达降低,miR-302b过表达可通过直接靶向Akt2减弱NF-κB和MMP-2的表达抑制癌细胞的侵袭和转移[12]。此外,miR-302b还可通过靶向调控E2F1增强乳腺癌细胞对顺铂的敏感性,降低细胞活性[13]。可见,miR-302b可通过调控多种靶基因或途径调控肿瘤的发生发展。
细胞周期紊乱是肿瘤细胞异常增殖的重要前提,CyclinD1是一种G1/S特异性周期蛋白,CDK2是一种在G1/S转变期和S期发挥重要作用的细胞周期蛋白依赖性激酶,两者异常表达均可导致食管癌细胞异常增殖[14-15]。除了细胞增殖失衡外,细胞凋亡异常也是肿瘤发生发展的重要机制。caspase-3是细胞凋亡过程中重要的凋亡执行蛋白,被活化后多以Cleaved caspase-3形式存在;Bax是公认的促凋亡基因,两者均在食管癌细胞凋亡过程中发挥重要作用[16]。本研究通过构建miR-302b-3p过表达和低表达的食管癌EC-109细胞,除了验证miR-302b-3p具有抑制EC-109细胞增殖和诱导细胞凋亡的作用外[4],还发现了miR-302b-3p过表达可使S期EC-109细胞百分比和CyclinD1及CDK2蛋白表达水平降低,使G0/G1期细胞百分比和细胞中Bax及Cleaved caspase-3蛋白表达水平升高。这一结果与GUO等[9]发现的miR-302b-3p抑制胃癌细胞增殖并诱导细胞凋亡的部分机制一致,提示miR-302b-3p可通过下调CyclinD1和CDK2表达诱导细胞周期阻滞于G0/G1期,抑制EC-109细胞增殖,同时还可上调Bax及Cleaved caspase-3表达诱导细胞凋亡。ECT2是一种与肿瘤发生发展关系密切的基因,被证实在非小细胞肺癌和乳腺癌等肿瘤组织中异常高表达,在调控细胞的增殖和凋亡等过程中具起重要作用[17]。研究[18]显示高表达的ECT2具有促进人恶性胶质瘤G1/S细胞周期进程的作用。近年来研究[5]显示:沉默ECT2表达可抑制乳腺癌细胞增殖并诱导细胞凋亡。本研究通过靶基因预测软件和双荧光素酶报告基因实验证实ECT2基因是miR-302b-3p的潜在靶基因,并且miR-302b-3p可负向调控ECT2蛋白的表达,提示食管癌中低表达的miR-302b-3p可能通过影响ECT2致癌功能发挥抑癌基因的作用。
综上所述,miR-302b-3p在食管癌发生发展过程中发挥抑癌基因的作用,可能通过靶向ECT2表达影响细胞中CyclinD1、CDK2、Bax和Cleaved caspase-3蛋白的表达介导食管癌EC-109细胞的增殖和凋亡过程。本研究进一步揭示了miR-302b-3p调控食管癌细胞增殖和凋亡的分子机制,为以miR-302b-3p为靶点的食管癌基因治疗提供了新的实验依据。
| [1] |
JIANG T, LIU JF, MU JX. Downregulation of microRNA-449a-5p promotes esophageal squamous cell carcinoma cell proliferation via cyclin D1 regulation[J]. Mol Med Rep, 2018, 18(1): 848-854. |
| [2] |
HU CM, LV L, PENG J, et al. MicroRNA-375 suppresses esophageal cancer cell growth and invasion by repressing metadherin expression[J]. Oncol Lett, 2017, 13(6): 4769-4775. DOI:10.3892/ol.2017.6098 |
| [3] |
ZHANG MX, ZHANG LM, CUI ML, et al. miR-302b inhibits cancer-related inflammation by targeting ERBB4, IRF2 and CXCR4 in esophageal cancer[J]. Oncotarget, 2017, 8(30): 49053-49063. |
| [4] |
ZHANG MX, YANG Q, ZHANG LM, et al. miR-302b is a potential molecular marker of esophageal squamous cell carcinoma and functions as a tumor suppressor by targeting ErbB4[J]. J Exp Clin Cancer Res, 2014, 33(1): 10. DOI:10.1186/1756-9966-33-10 |
| [5] |
肖安, 彭蓉蓉. ECT2基因沉默调控人乳腺癌细胞增殖和凋亡及其机制探讨[J]. 肿瘤, 2017, 37(6): 594-603. |
| [6] |
TAN HY, WANG XS, YANG XG, et al. Oncogenic role of epithelial cell transforming sequence 2 in lung adenocarcinoma cells[J]. Exp Ther Med, 2016, 12(4): 2088-2094. DOI:10.3892/etm.2016.3584 |
| [7] |
梁冰, 刘灿, 王峰. miRNA-138在TGF-β1诱导的乳腺癌上皮间质转化发生中的作用[J]. 郑州大学学报:医学版, 2017, 52(6): 753-757. |
| [8] |
刘襄艳, 侯桂琴, 高盼, 等. miR-199 a-3p对Eca109和KYSE450细胞周期和迁移的影响[J]. 郑州大学学报:医学版, 2017, 52(6): 668-672. |
| [9] |
GUO B, ZHAO ZH, WANG Z, et al. MicroRNA-302b-3p suppresses cell proliferation through AKT pathway by targeting IGF-1R in human gastric cancer[J]. Cell Physiol Biochem, 2017, 42(4): 1701-1711. DOI:10.1159/000479419 |
| [10] |
HUANG J, HE Y J, MCLEOD H L, et al. miR-302b inhibits tumorigenesis by targeting EphA2 via Wnt/β-catenin/EMT signaling cascade in gastric cancer[J]. BMC Cancer, 2017, 17(1): 886. DOI:10.1186/s12885-017-3875-3 |
| [11] |
KHODAYARI N, MOHAMMED K A, LEE H, et al. MicroRNA-302b targets Mcl-1 and inhibits cell proliferation and induces apoptosis in malignant pleural mesothelioma cells[J]. Am J Cancer Res, 2016, 6(9): 1996-2009. |
| [12] |
WANG L M, YAO J Y, SUN H F, et al. miR-302b suppresses cell invasion and metastasis by directly targeting AKT2 in human hepatocellular carcinoma cells[J]. Tumor Biol, 2016, 37(1): 847-855. DOI:10.1007/s13277-015-3330-5 |
| [13] |
CATALDOA C, CHEUNG D G, BALSARI A, et al. miR-302b enhances breast cancer cell sensitivity to cisplatin by regulating E2F1 and the cellular DNA damage response[J]. Oncotarget, 2016, 7(1): 786-797. |
| [14] |
ZHAO L, WEI Z B, YANG C Q, et al. Effects of PLCE1 gene silencing by RNA interference on cell cycling and apoptosis in esophageal carcinoma cells[J]. Asian Pac J Cancer Prev, 2014, 15(13): 5437-5442. DOI:10.7314/APJCP.2014.15.13.5437 |
| [15] |
ZENG L S, YANG X Z, WEN Y F, et al. Overexpressed HDAC4 is associated with poor survival and promotes tumor progression in esophageal carcinoma[J]. Aging(Albany NY), 2016, 8(6): 1236-1249. |
| [16] |
LIU L, ZUO J, WANG G. Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis in Ec9706 and Eca109 esophageal carcinoma cells[J]. Oncol Lett, 2017, 14(4): 4391-4395. DOI:10.3892/ol.2017.6712 |
| [17] |
ZHOU S J, WANG P, SU X L, et al. Correction: High ECT2 expression is an independent prognostic factor for poor overall survival and recurrence-free survival in non-small cell lung adenocarcinoma[J]. PLoS One, 2018, 13(4): e0196354. DOI:10.1371/journal.pone.0196354 |
| [18] |
ZHU C, WANG T T, CHENG S Y, et al. Epithelial cell transforming sequence 2(Ect2) promotes G1/S cell cycle progression[J]. Clin Cancer Res, 2007, 13: C77. |
 2019, Vol. 45
2019, Vol. 45
