扩展功能
文章信息
- 韩耀伦, 王璐, 马欣
- HAN Yaolun, WANG Lu, MA Xin
- 牙髓炎模型大鼠血清褪黑素水平及其对牙髓炎症反应的抑制作用
- Levels of serum melatonin in rats with pulpitis and its inhibitory effect on dental inflammation
- 吉林大学学报(医学版), 2019, 45(06): 1334-1339
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1334-1339
- 10.13481/j.1671-587x.20190624
-
文章历史
- 收稿日期: 2019-02-16
牙髓炎是口腔多发病和常见病,以剧烈、难以忍受的疼痛为主要表现,严重影响患者的身心健康。牙髓炎的主要病因为细菌导致的牙髓感染,牙髓组织的免疫反应在牙髓炎的发生过程中发挥重要作用[1]。核苷酸结合寡聚化结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3,NLRP3)炎症体包含NLRP3蛋白和未活化的含半胱氨酸的天冬氨酸蛋白水解酶(cysteine aspartate proteolytic enzyme 1,caspase-1),是识别危险信号的内源性多蛋白复合物;通过NLRP3炎症体可诱导caspase-1自剪切活化,识别胞内危险信号;炎症caspase-1的活化使不成熟的白细胞介素1β(interleukin-1β,IL-1β)前体剪切成为具有活性的IL-1β,从而参与炎症反应[2]。在实验性急性牙髓炎大鼠牙髓组织中NLRP3蛋白呈阳性表达,NLRP3可能在急性牙髓炎的发生发展过程中发挥重要作用[3]。褪黑素具有调节免疫功能的作用[4-5],可通过抑制NLRP3炎性小体在蛛网膜下腔出血小鼠中发挥脑保护作用[6]。但褪黑素在牙髓炎中的作用及其可能机制尚不清楚。本研究观察牙髓炎大鼠血清褪黑素水平及其对NLRP3/caspase-1信号通路的影响,探讨褪黑素在牙髓炎中的作用及其可能机制。
1 材料与方法 1.1 实验动物、主要试剂和仪器8周龄SD大鼠130只,雌雄各半,健康,清洁级,购自中国科学院微生物研究所,动物许可证号:SYXK(京)2014-0032。褪黑素、RIPA裂解液、BCA蛋白浓度测定试剂盒和ELISA试剂盒(美国Sigma公司),RT-PCR试剂盒、氯仿和异丙醇(美国Invitrogen公司),NLRP3抗体和caspase-1抗体(美国Abcam公司)。7500荧光定量PCR仪(美国LIGHT ABI公司),高速涡轮机(日本NSK公司)。
1.2 牙髓炎大鼠模型的建立水合氯醛腹腔注射麻醉大鼠,将大鼠仰卧固定在动物手术台上,采用镊子将大鼠上颌撑开,暴露上颌磨牙,酒精消毒上颌,采用高速涡轮机005号球钻在大鼠左侧上颌第一磨牙和第二磨牙颌面开髓,深度约1 mm,牙本质透红后采用金属探针加压形成穿髓孔开放髓腔,使髓腔在口腔中暴露。牙髓炎建模成功的标准为牙髓血管扩张充血,冠髓部可见大量白细胞浸润,呈现典型的牙髓炎变化。
1.3 大鼠分组取40只大鼠作为对照组(牙髓不予处理),取90只大鼠建立牙髓炎模型。取10只对照组大鼠和30只牙髓炎大鼠进行牙髓组织HE染色和血清IL-1β和褪黑素水平测定,其中30只牙髓炎大鼠根据随机数字法分为牙髓炎1 d组、牙髓炎3 d组和牙髓炎5 d组,每组10只。剩下的30只对照组大鼠和60只牙髓炎大鼠进行牙髓组织中IL-1β水平和NLRP3及caspase-1 mRNA和蛋白水平测定,且60只牙髓炎大鼠根据随机数字法分为牙髓炎组和牙髓炎+褪黑素组,每组30只。
1.4 HE染色检测各组大鼠牙髓组织的病理形态表现将对照组和牙髓炎1 d组、3 d组和5 d组大鼠分别在相应时间点以水合氯醛麻醉成功后,开胸,心脏取血,进行血清指标检查,采用生理盐水静滴至肝脏变白,以多聚甲醛固定。采用组织剪于腭中线处分开左、右上颌骨,取出部分上颌骨和上颌磨牙,在多聚甲醛中固定12 h,置入EDTA脱钙,完全脱钙后,梯度酒精脱水至蜡,包埋,切成4 μm厚切片,二甲苯脱蜡,梯度酒精脱水,苏木精染色,盐酸乙醇分化,乙醇脱水,伊红复染,乙醇脱水,二甲苯透明,中性树胶封片,显微镜下观察大鼠牙髓组织的成牙本质层细胞形态表现,血管扩张及炎性细胞情况。
1.5 各组大鼠血清IL-1β和褪黑素水平测定将各组大鼠心脏血液离心(2 000 r·min-1),留取血清,采用ELISA法测定各组大鼠血清IL-1β和褪黑素水平,单位为ng·L-1。
1.6 各组大鼠的给药方式牙髓炎+褪黑素组大鼠于建立牙髓炎模型术后立即腹腔注射褪黑素(10 mg·kg-1),对照组和牙髓炎组大鼠腹腔注射等量生理盐水;24 h后取对照组、牙髓炎组和牙髓炎+褪黑素组各10只大鼠行后续检测。
1.7 各组大鼠牙髓组织中IL-1β水平测定水合氯醛麻醉成功后,活体取出上颌骨,剥离大鼠磨牙,取牙髓组织标本,采用ELISA法测定大鼠牙髓组织中IL-1β水平,实验步骤按照ELISA试剂盒说明书操作,单位为ng·L-1。
1.8 各组大鼠牙髓组织中NLRP3和caspase-1 mRNA表达水平测定水合氯醛麻醉成功后活体取出上颌骨,剥离大鼠磨牙,去净牙根周围软组织,组织剪剪碎磨牙,在液氮中研磨值粉末,将研磨好的粉末转移到RNA酶灭活EP管中提取mRNA。将mRNA反转录为cDNA,以GAPDH为内参照,按照RT-PCR说明书进行PCR反应,反应条件:95℃、5 min,95℃、30 s、60℃、1 min、72℃、30 s,共40个循环,72℃、1 min。NLRP3引物序列:上游引物为5′-CAGCGATGAAGACGCGAGAG-3′,下游引物为5′-AGAGATATGGCACGAAAGCTATCCA-3′。caspase-1引物序列:上游引物为5′-ACTGCTACACCTGTTGCGCCTCA-3′,下游引物为5′-CTGCCGACGCAGGAAATTC-3′。以2-ΔΔCt法计算大鼠髓组织中NLRP3和caspase-1 mRNA表达水平。
1.9 各组大鼠牙髓组织中NLRP3和caspase-1蛋白表达水平测定水合氯醛麻醉成功后活体取出上颌骨,剥离大鼠磨牙,去净牙根周围软组织,剪碎磨牙,在液氮中研磨至粉末,将研磨好的粉末转移至EP管中,加入RIPA裂解液,提取牙髓组织总蛋白,BCA法测定总蛋白浓度,采用Western blotting法测定牙髓组织中NLRP3和caspase-1蛋白水平。以β-actin为内参照,经上样、电泳、转膜和封闭,加入一抗(NLRP3抗体为1:100,caspase-1抗体为1:100)过夜孵育,加入二抗孵育1 h。采用Odyssey成像仪扫膜保持图像,采用Image J软件分析蛋白灰度值。NLRP3和caspase-1蛋白表达水平=NLRP3和caspase-1蛋白条带灰度值/β-actin条带灰度值。
1.10 统计学分析采用SPSS20.0统计软件进行统计学分析。各组大鼠血清IL-1β和褪黑素水平、牙髓组织中IL-1β水平、牙髓组织中NLRP3和caspase-1mRNA及蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠牙髓组织形态表现对照组大鼠牙髓组织无炎性渗出、未见炎细胞浸润,无血管扩张。牙髓炎1 d组大鼠牙髓穿髓点下方见中性粒细胞浸润,成牙本质细胞排列紊乱,冠髓血管扩张明显;牙髓炎3 d组大鼠牙髓穿髓点下方见大量中性粒细胞聚集,成牙本质细胞变性坏死,整个牙髓组织见血管充血扩张明显;牙髓炎5 d组大鼠牙髓穿髓点下方有脓腔形成,脓腔周围有纤维组织形成,冠髓基本坏死,坏死周围有大量炎性细胞聚集。见图 1(插页五)。
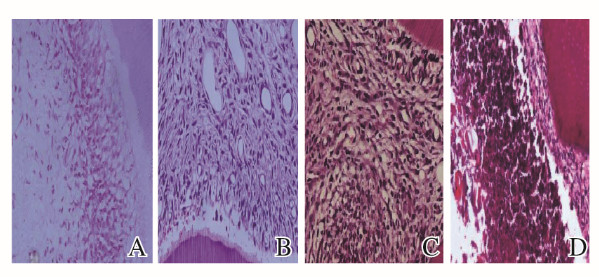
|
| A:Control group; B:Pulpitis 1 d group; C:Pulpitis 3d group; D:Pulpitis 5 d group. 图 1 各组大鼠牙髓组织形态表观(HE,×200) Fig. 1 Morphology of pulp tissue of rats in various groups(HE, ×200) |
|
|
各组大鼠血清IL-1β和褪黑素水平比较差异有统计学意义(P>0.05),其中牙髓炎1 d组和牙髓炎3 d组大鼠血清IL-1β水平高于对照组(P < 0.05),褪黑素水平低于对照组(P < 0.05);牙髓炎5 d组大鼠血清IL-1β和褪黑素水平与对照组比较差异无统计学意义(P>0.05);牙髓炎3 d组大鼠血清IL-1β水平低于牙髓炎1 d组(P < 0.05),褪黑素水平高于牙髓炎1 d组(P < 0.05)。见表 1。
| [n=10, x±s, ρB/(ng·L-1)] | |||||||||||||||||||||||||||||
| Group | IL-1β | Melatonin | |||||||||||||||||||||||||||
| Control | 18.42±1.94 | 48.25±2.41 | |||||||||||||||||||||||||||
| Pulpitis 1 d | 46.52±1.86* | 25.32±2.37* | |||||||||||||||||||||||||||
| Pulpitis 3 d | 28.73±1.79*△ | 42.15±2.31*△ | |||||||||||||||||||||||||||
| Pulpitis 5 d | 19.24±1.92 | 47.41±2.42 | |||||||||||||||||||||||||||
| * P < 0.05 compared with control group; △ P < 0.05 compared with pulpitis 1 d group. | |||||||||||||||||||||||||||||
牙髓炎组和牙髓炎+褪黑素组大鼠牙髓组织中IL-1β水平高于对照组(P < 0.05),牙髓炎+褪黑素组大鼠牙髓组织中IL-1β水平低于牙髓炎组(P < 0.05)。见表 2。
| [n=10, x±s, ρB/(ng·L-1)] | |||||||||||||||||||||||||||||
| Group | IL-1β | ||||||||||||||||||||||||||||
| Control | 4.98±1.45 | ||||||||||||||||||||||||||||
| Pulpitis | 27.53±1.62* | ||||||||||||||||||||||||||||
| Pulpitis+melatonin | 14.37±1.53*△ | ||||||||||||||||||||||||||||
| * P < 0.05 compared with control group;△ P < 0.05 compared with pulpitis group. | |||||||||||||||||||||||||||||
牙髓炎组和牙髓炎+褪黑素组大鼠牙髓组织中NLRP3和caspase-1 mRNA表达水平高于对照组(P < 0.05);牙髓炎+褪黑素组大鼠牙髓组织中NLRP3和caspase-1 mRNA表达水平低于牙髓炎组(P < 0.05)。见表 3。
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | NLRP3 mRNA | Caspase-1 mRNA | |||||||||||||||||||||||||||
| Control | 0.28±0.09 | 0.32±0.11 | |||||||||||||||||||||||||||
| Pulpitis | 0.72±0.13* | 0.84±0.15* | |||||||||||||||||||||||||||
| Pulpitis+melatonin | 0.49±0.14*△ | 0.51±0.12*△ | |||||||||||||||||||||||||||
| * P < 0.05 compared with control group;△ P < 0.05 compared with pulpitis group. | |||||||||||||||||||||||||||||
牙髓炎组和牙髓炎+褪黑素组大鼠牙髓组织中NLRP3和caspase-1蛋白表达水平高于对照组(P < 0.05);牙髓炎+褪黑素组大鼠牙髓组织中NLRP3和caspase-1蛋白表达水平低于牙髓炎组(P < 0.05)。见表 4和图 2。
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | NLRP3 protein | Caspase-1 protein | |||||||||||||||||||||||||||
| Control | 0.23±0.06 | 0.14±0.05 | |||||||||||||||||||||||||||
| Pulpitis | 0.95±0.12* | 0.98±0.14* | |||||||||||||||||||||||||||
| Pulpitis+melatonin | 0.78±0.11*△ | 0.73±0.12*△ | |||||||||||||||||||||||||||
| * P < 0.05 compared with control group;△ P < 0.05 compared with pulpitis group. Lane 1: Control group;Lane 2: Pulpitis group;Lane 3: Pulpitis+melatonin group. | |||||||||||||||||||||||||||||

|
| * P < 0.05 compared with control group; △ P < 0.05 compared with pulpitis group. Lane 1: Control group; Lane 2: Pulpitis group; Lane 3: Pulpitis+melatonin group. 图 2 Western blotting法检测各组大鼠牙髓组织中NLRP3和caspase-1蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of NLRP3 and caspase-1 proteins in dental pulp tissue of rats in various groups detected by Western blotting method |
|
|
牙髓组织由成牙本质细胞和成纤维细胞等多种细胞组成,成牙本质细胞和成纤维细胞参与牙髓组织的免疫防御过程;牙髓组织的最外层为成牙本质细胞,成牙本质细胞最早接触侵入牙本质小管的牙本质碎屑、细菌和细菌分解产物[7]。在牙龋受损时成牙本质细胞可表达IL-1β和TNF-α等细胞因子,并同时分泌趋化因子,聚集树突状细胞,启动免疫反应。牙髓组织的主体细胞为成纤维细胞,表面表达多种Toll样受体,可以识别病原体并分泌促炎因子[8-9]。IL-1β是重要的促炎因子之一,在牙髓炎时其表达量升高,参与牙髓炎的免疫反应[10]。
NLRP3炎症体在机体固有免疫防御中发挥重要作用,在牙髓-牙本质复合体受到损伤或外源性刺激时可触发机体防御活动,对外界刺激做出反应[11]。NLRP3在固有免疫反应中参与的免疫调节通路被激活后进一步活化caspase-1,活化的caspase-1调节IL-1β的合成和释放,参与免疫炎症反应[12-13]。在牙髓炎中,模式识别受体识别病原相关分子模式激活固有免疫系统,抵抗病原微生物的入侵,NLRP3属于胞质内模式识别受体,NLRP3炎症体通过激活caspase-1促进IL-1β的表达,参与牙髓炎的发生发展[14]。本研究结果显示:牙髓炎大鼠血清IL-1β水平升高,在牙髓炎1 d时其水平最高,随后逐渐降低;在牙髓炎大鼠牙髓组织中IL-1β水平升高,NLRP3和caspase-1mRNA及蛋白表达水平也升高,表明牙髓炎时病原菌可能激活NLRP3,进一步活化caspase-1,调节IL-1β的合成和释放,参与牙髓炎的免疫炎症反应。
在牙周炎中,NLRP3被激活形成炎性小体,炎性小体活化caspase-1,活化的caspase-1进一步活化IL-1β前体,释放IL-1β清除病原微生物,IL-1β过量表达可造成牙髓炎、牙周炎和根尖周炎等,调控NLRP3炎性小体及炎性小体下游炎性因子的表达可能成为治疗牙周炎的新思路[15-16]。褪黑素由松果体分泌产生,具有促进睡眠和调节免疫功能等作用[17]。在机体发生急性应激反应时褪黑素具有免疫抑制作用;褪黑素可影响巨噬细胞/小神经胶质细胞数量、免疫功能和吞噬活性[18-19]。褪黑素还具有参与炎症调控的作用,可通过多种途径抑制炎症反应[20]。褪黑素可能通过抑制NLRP3炎症小体表达抑制炎症反应。贾天明等[21]发现:癫痫模型大鼠海马组织中NLRP3、caspase-1和IL-1β表达水平升高,NLRP3炎性小体被激活,褪黑素可通过抑制海马组织中NLRP3、caspase-1和IL-1β表达,抑制NLRP3炎性小体激活从而发挥脑保护作用。褪黑素是否通过抑制NLRP3炎性小体激活抑制牙髓炎的免疫炎症反应尚不清楚。本研究结果显示:牙髓炎大鼠给予褪黑素治疗后,牙髓组织中NLRP3、caspase-1和IL-1β水平降低,表明褪黑素可能通过抑制NLRP3激活,从而抑制caspase-1活化,降低IL-1β的分泌和释放,从而抑制牙髓炎的免疫炎症反应。
综上所述,褪黑素在牙髓炎的发生发展中发挥作用,给予褪黑素治疗可通过抑制NLRP3/caspase-1通路抑制牙髓炎的免疫炎症反应。
| [1] |
LARSEN T, FIEHN N E. Dental biofilm infections-an update[J]. APMIS, 2017, 125(4): 376-384. DOI:10.1111/apm.12688 |
| [2] |
WU D, SHI L, LI PY, et al. Intermedin1-53protects cardiac fibroblasts by inhibitingNLRP3 inflammasome activation during sepsis[J]. Inflammation, 2018, 41(2): 505-514. DOI:10.1007/s10753-017-0706-2 |
| [3] |
王培娜, 王海婧, 蒋文凯, 等. NLRP3炎症体在小鼠实验性急性牙髓炎中的表达[J]. 牙体牙髓牙周病学杂志, 2015, 25(5): 259-262. |
| [4] |
CHEN YN, ZHAO Q, SUN YJ, et al. Melatonininducesanti-inflammatoryeffects via endoplasmic reticulum stress in RAW264.7 macrophages[J]. Mol Med Rep, 2018, 17(4): 6122-6129. |
| [5] |
DEHGHAN F, SHAHROKHI N, KHAKSARI M, et al. Does the administration ofmelatoninduring post-traumatic brain injury affect cytokine levels?[J]. Inflammopharmacology, 2018, 26(4): 1017-1023. DOI:10.1007/s10787-017-0417-1 |
| [6] |
毛崇丹, 甄恩迪, 刘阳阳, 等. 褪黑激素抑制NLRP3炎性小体减轻小鼠蛛网膜下腔出血早期脑损伤[J]. 中华神经外科疾病研究杂志, 2017, 16(5): 417-421. |
| [7] |
SUGIUCHI A, SANO Y, FURUSAWA M, et al. Human dental pulp cells express cellular markers for inflammation and hard tissue formation in response to bacterial Information[J]. J Endod, 2018, 44(6): 992-996. DOI:10.1016/j.joen.2018.02.022 |
| [8] |
SONG FF, SUN HL, WANG YK, et al. Pannexin3 inhibits TNF-α-induced inflammatory response by suppressing NF-κB signalling pathway in human dental pulp cells[J]. J Cell Mol Med, 2017, 21(3): 444-455. DOI:10.1111/jcmm.12988 |
| [9] |
HUANG SH, SONG Z, HUANG QT, et al. AIM2 inflammasome is critical for dsDNA-Induced IL-1β secretion in human dental pulp cells[J]. Inflammation, 2018, 41(2): 409-417. DOI:10.1007/s10753-017-0697-z |
| [10] |
GHATTAS AYOUB C, AMINOSHARIAE A, BAKKAR M, et al. Comparison of IL-1β, TNF-α, hBD-2, and hBD-3 expression in the dental pulp of smokers versus nonsmokers[J]. J Endod, 2017, 43(12): 2009-2013. DOI:10.1016/j.joen.2017.08.017 |
| [11] |
HU Q, ZHANG T, YI L, et al. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells[J]. Biofactors, 2018, 44(2): 123-136. DOI:10.1002/biof.1395 |
| [12] |
ZOU P, LIU XX, LI G, et al. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1[J]. Mol Med Rep, 2018, 17(2): 3212-3217. |
| [13] |
JIANG WK, LV H, WANG HJ, et al. Activation of the NLRP3/Caspase-1 inflammasome in human dental pulp tissue and human dental pulp fibroblasts[J]. Cell Tissue Res, 2015, 361(2): 541-555. DOI:10.1007/s00441-015-2118-7 |
| [14] |
王雪纯, 薛丽英. NOD1, NOD2和NLRP3炎症小体与牙髓炎[J]. 临床与病理杂志, 2017, 37(4): 849-854. |
| [15] |
LEE S I, KANG S K, JUNG H J, et al. Muramyl dipeptide activates human beta defensin 2 and pro-inflammatory mediators through Toll-like receptors and NLRP3 inflammasomes in human dental pulp cells[J]. Clin Oral Invest, 2015, 19(6): 1419-1428. DOI:10.1007/s00784-014-1361-8 |
| [16] |
王娜娜, 陈莉丽, 丁佩惠. 核苷酸结合寡聚化结构域样受体家族热蛋白结构域3炎性小体与牙周炎[J]. 国际口腔医学杂志, 2017, 44(4): 484-487. |
| [17] |
JI M H, XIA D G, ZHU L Y, et al. Short- and long-term protective effects of melatonin in a mouse model of sepsis-associated encephalopathy[J]. Inflammation, 2018, 41(2): 515-529. DOI:10.1007/s10753-017-0708-0 |
| [18] |
BERKIKS I, BENMHAMMED H, MESFIOUI A, et al. Postnatal melatonin treatment protects against affective disorders induced by early-life immune stimulation by reducing the microglia cell activation and oxidative stress[J]. Int J Neurosci, 2018, 128(6): 495-504. DOI:10.1080/00207454.2017.1398156 |
| [19] |
ZHANG Y, LI X, GRAILER J J, et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3inflammasome[J]. J Pineal Res, 2016, 60(4): 405-414. DOI:10.1111/jpi.12322 |
| [20] |
XU LJ, ZHANG LX, WANG ZF, et al. Melatonin suppresses estrogen deficiency-induced osteoporosis and promotes osteoblastogenesis by inactivating the NLRP3 inflammasome[J]. Calcif Tissue Int, 2018, 103(4): 400-410. DOI:10.1007/s00223-018-0428-y |
| [21] |
贾天明, 李月琴, 张晓莉, 等. 核苷酸结合寡聚化结构域样受体蛋白3炎性小体在癫痫大鼠模型中的表达及褪黑素对其的影响[J]. 中华实用儿科临床杂志, 2018, 33(12): 913-917. DOI:10.3760/cma.j.issn.2095-428X.2018.12.009 |
 2019, Vol. 45
2019, Vol. 45


