扩展功能
文章信息
- 边红恩, 陈团营
- BIAN Hongen, CHEN Tuanying
- 沙参麦冬汤对新生大鼠支气管肺发育不良的保护作用及其机制
- Protective effect of Radix Salviae Miltiorrhizae Decoction on bronchopulmonary dysplasia in neonatal rats and its mechanism
- 吉林大学学报(医学版), 2019, 45(06): 1327-1333
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1327-1333
- 10.13481/j.1671-587x.20190623
-
文章历史
- 收稿日期: 2019-01-04
支气管肺发育不良(bronchopulmonary dysplasia,BPD)是常见于早产儿尤其是出生体质量极低新生儿的一种慢性肺疾病。早产儿由于肺发育不成熟,对缺氧缺血耐受性差,常需接受氧疗,BPD是早产儿长时间吸入高体积分数氧(高氧)治疗的常见严重并发症之一[1]。在出生体质量不足1 kg的早产儿中,BPD的发生率超过50% [2]。患儿常见遗留不同程度的肺功能障碍,也增加了发生脑性瘫痪和智力低下的风险[3]。BPD的发生主要与肺发育不成熟、感染、氧疗、机械通气和氧自由基等因素有关[4-6]。20世纪90年代,糖皮质激素、肺表面活性物质和肺保护性通气已成为治疗和预防BPD的常规策略,但这种疗法可引起严重的神经运动发育障碍、血糖升高和肠穿孔等,并能增加感染机会[7-8]。因此探讨BPD的高效低毒不良反应治疗药物或方法已成为热点研究[9]。维生素A与维生素D在肺组织发育和预防BPD中起重要作用[10],槲皮素能够减轻新生小鼠的高氧肺损伤,对BPD有一定的疗效[11]。本研究采用高氧刺激方法建立新生鼠BPD模型,观察沙参麦冬汤加减对新生大鼠BPD的影响及其对免疫细胞因子等的影响。
1 材料与方法 1.1 实验动物、主要试剂和仪器200只新生SPF级Wistar大鼠(出生24 h内),雌雄不限,出生体质量4~7 g,购自河南省实验动物生产有限公司,动物合格证号:SKXY(豫)2012-0002。地塞米松磷酸钠注射液购自瑞阳制药有限公司(国药准字H37022031),一步法动物组织活性蛋白提取试剂盒C500006-0020购自美国Sangon Biotech公司,G1100伊红染色液和G1140苏木素染色液购自北京索莱宝科技有限公司,大鼠丙二醛(malondialdehyde,MDA)、超氧化物歧化酶(superoxidedismutase,SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)、白细胞介素1(interleukin-1,IL-1)、白细胞间介素10(interleukin - 10,IL-10)和肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)ELISA试剂盒购自美国Biolegend有限公司,鼠抗人促凋亡蛋白Bax、抗凋亡蛋白Bcl-2和caspase-3单克隆抗体购自美国Santa Cruz公司,鼠抗人GADPH单克隆抗体购自美国Santa Cruz公司。JJ300型电子天平购自广州湘仪机电设备有限公司,LDZX-50FBS立式压力蒸汽灭菌器购自上海申安医疗器械厂,YS100光学显微镜购自日本Nikon公司,UV-1800 PC紫外分光光度计购自上海析谱仪器有限公司,TDL5M低温高速离心机购自上海聚莱实验仪器有限公司。
1.2 沙参麦冬汤的制备沙参麦冬汤(北沙参18 g、玉竹12 g、麦冬18 g、天花粉9 g、扁豆9 g、桑叶9 g、生甘草6 g、地骨皮18 g)按《温病条辨》原方剂量等比例扩大2倍组成,制成生药浓度为2.4 g·mL-1的中药原液作为高剂量沙参麦冬汤组,生药浓度为0.6 g·mL-1的中药原液作为低剂量沙参麦冬汤组,高压灭菌4℃保存;
1.3 动物模型分组和给药将200只大鼠幼鼠按随机数字法分为对照组、模型组、地塞米松组、低和高剂量沙参麦冬汤组,每组40只。实验操作严格依据中国科学技术委员会提出的《实验动物管理条例》进行,均饲养于干净的独立通气笼盒(individualventilatedcages, IVC)中,每日供应充足的干净水源和标准动物饲料,室内环境控制在12 h /12 h日夜交替,温度控制为20℃~25℃,湿度控制范围为55% ~60%。各组大鼠体质量、饲养方式和鼠龄比较差异无统计学意义(P>0.05),具有可比性。除对照组40只大鼠外,将其余160只新生鼠于出生12 h内连同代母鼠置于氧箱中,持续吸入高氧,维持吸入氧体积分数(FiO2)为900 mL·L-1,二氧化碳(CO2)体积分数<5 mL·L-1 (钠石灰吸收CO2),温度25℃~27℃,湿度50%~70%(变色硅胶吸收水蒸气),每24 h定时开箱0.5 h [11]。造模成功后分为模型组、低和高剂量沙参麦冬汤组及地塞米松组。沙参麦冬汤组和地塞米松组大鼠在造模的同时灌胃给予6.00和24.00mg·kg-1沙参麦冬汤,地塞米松组大鼠于给药第1天起腹腔注射给予0.75 μg·kg-1地塞米松注射液。对照组大鼠置于空气中(FiO2为210 mL·L-1, 具体方法及实验控制因素同模型组),对照组大鼠给予等量生理盐水,各组持续给药21 d。
1.4 苏木素-伊红(HE)染色观察各组大鼠肺组织形态表现给药21 d后, 每组随机选取10只大鼠,麻醉后打开胸腔,分离肺组织。将肺组织块置于10%甲醛溶液中固定,进行常规石蜡切片,厚度为4 μm,经HE染色、中性树胶封片后于LEICA DMLB型光学显微镜下观察大鼠肺组织形态表观,并观察大鼠肺组织中放射状肺泡计数(radical alveolar counts, RAC),每只大鼠于不同时问点抽取HE染色切片5张,每张切片于光镜下计算RAC,即从呼吸性细支气管中心至远端胸膜引一垂直线上的肺泡数量,选取5个视野,计算各组大鼠RAC平均值。
1.5 各组大鼠肺湿质量和干质量的检测给药21 d后, 每组随机选取10只大鼠,麻醉后打开胸腔,分离全部肺组织,用预冷的PBS冲洗表面血液,以滤纸轻轻吸干PBS,称取湿质量(W),将其置于75℃的烤箱中烘烤72 h,取出称量肺干质量(D),两者比值为肺W/D值,W/D值可评定急性炎性反应和肺水肿程度。
1.6 各组大鼠肺组织中MDA、SOD和GSH-Px水平测定每组随机选取10只大鼠,麻醉后打开胸腔,分离左肺组织,用预冷的生理盐水洗净肺组织,吸干水分,称1 g肺组织剪碎,于冰盒内超声粉碎匀浆,制成10%组织匀浆,4℃低温离心机3 000 r·min-1离心10 min后取上清液, 按照南京建成生物工程研究所提供试剂盒要求,分别采用硫代巴比妥酸法、亚硝酸盐法和二硝基苯甲酸法用分光光度剂依次在532、550和422 nm处测定其吸光度(A)值,分别计算目标指标水平。目标指标水平=(测定管A值-测定空白管A值)/(标准管A值-标准空白管A值)×样品浓度×样品测试前稀释倍数。
1.7 各组大鼠肺泡灌洗液中IL-1、IL-10和TNF-α水平测定称l g肺组织剪碎,于冰盒内超声粉碎匀浆,制成10%组织匀浆,4℃低温离心机3 000 r·min-1离心10 min后取上清液, 采用ELISA试剂盒方法检测各组大鼠肺泡灌洗液中IL-1、IL-10和TNF-α水平。
1.8 各组大鼠肺组织中Bax、Bcl-2和caspase-3蛋白表达水平测定给药21 d后, 每组随机选取10只大鼠,麻醉后打开胸腔, 无菌操作下取出肺组织于蛋白裂解缓冲液中匀浆, 用一步法动物组织活性蛋白提取试剂盒分离提取大鼠肺总蛋白。总蛋白浓度测定符合要求后,蛋白于SDS-PAGE胶上进行电泳分离并转膜。5%脱脂牛奶封闭,于4 ℃摇床中一抗(1:1 000)孵育过夜,PBS洗膜3次,二抗(1:5 000)孵育1.5 h, PBS洗膜3次,ECL化学发光试剂盒显像。利用化学发光荧光成像仪的MI软件中的图像分析功能对条带灰度进行分析,将目的蛋白条带与内参条带灰度值的比值作为半定量结果。
1.9 统计学分析采用SPSS19.0统计软件进行统计学分析。各组大鼠肺组织W/D值、RAC、肺组织中MDA、SOD和GSH-Px水平、大鼠肺泡灌洗液中细胞炎性因子IL-1、IL-10和TNF-α水平以及大鼠肺组织中Bax、Bcl-2和caspase-3蛋白表达水平以x±s表示,各组数据均呈正态分布,多组间样本均数比较采用单因素方差分析,组间样本均数比较采用t检验。以P < 0.05为表示差异有统计学意义。
2 结果 2.1 各组大鼠肺组织W/D值与对照组比较,模型组大鼠肺组织W/D值明显升高,可见明显肺水肿;与模型组比较,地塞米松组、低和高剂量沙参麦冬汤组大鼠肺水肿情况均有不同程度改善,肺组织W/D值明显降低(P < 0.05或P < 0.01),且以高剂量沙参麦冬汤组肺组织W/D值降低最明显。见表 1。
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | Dose | W/D value | |||||||||||||||||||||||||||
| Control | - | 4.83±0.22 | |||||||||||||||||||||||||||
| Model | - | 5.98±0.31* | |||||||||||||||||||||||||||
| Dexamethasone | 0.75 μg·kg-1 | 5.12±0.81△ | |||||||||||||||||||||||||||
| Low dose of Radix Salviae Miltiorrhizae Decoction | 6.00 mg·kg-1 | 5.47±0.62△ | |||||||||||||||||||||||||||
| High dose of Radix Salviae Miltiorrhizae Decoction | 24.00 mg·kg-1 | 4.95±0.25△△ | |||||||||||||||||||||||||||
| * P < 0.01 compared with control group; △ P < 0.05, △△ P < 0.01 compared with model group.“-”:No data. | |||||||||||||||||||||||||||||
对照组大鼠随鼠龄增加肺组织发育逐渐成熟,RAC逐渐增多,大鼠肺泡结构规整,大小均匀,肺泡间隔正常。模型组大鼠高氧暴露21 d后肺泡腔扩大、结构简化,间隔增厚,RAC较对照组明显减少(t=3.989, P < 0.05),炎症细胞浸润。地塞米松组大鼠肺泡结构紊乱情况较模型组减轻,RAC有所增多(P < 0.05),肺泡腔有所缩小;高剂量沙参麦冬汤组大鼠肺泡结构紊乱情况较模型组减轻,RAC明显增多(P < 0.01),肺泡腔缩小,肺组织充血、水肿及炎症程度减轻;与模型组比较,低剂量沙参麦冬汤组大鼠肺组织充血、水肿及炎症程度减轻,RAC略有增加(P < 0.05),肺组织结构未见明显改善。见图 1(插页五)和表 2。
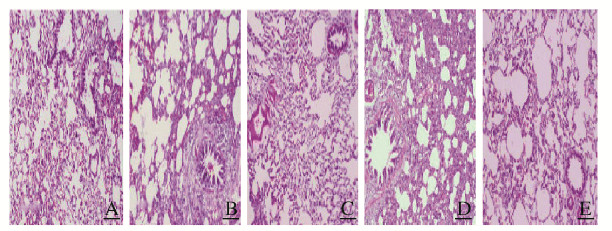
|
| A:Control group; B: Model group; C: Dexamethasone group; D: High dose of Radix Salviae Miltiorrhizae Decoction group; E: Low dose of Radix Salviae Miltiorrhizae Decoction group. 图 1 各组小鼠肺组织病理形态表现(×400) Fig. 1 Pathomorphology of lung tissue of rats in various groups(×400) |
|
|
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | Dose | RAC | |||||||||||||||||||||||||||
| Control | - | 13.3±1.2 | |||||||||||||||||||||||||||
| Model | - | 6.8±1.1* | |||||||||||||||||||||||||||
| Dexamethasone | 0.75 μg·kg-1 | 9.1±1.1△ | |||||||||||||||||||||||||||
| Low dose of Radix Salviae Miltiorrhizae Decoction | 6.00 mg·kg-1 | 8.2±1.0△ | |||||||||||||||||||||||||||
| High dose of Radix Salviae Miltiorrhizae Decoction | 24.00 mg·kg-1 | 11.5±1.4△△ | |||||||||||||||||||||||||||
| * P < 0.01 compared with control group; △ P < 0.05, △△ P < 0.01 compared with model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组大鼠肺组织中MDA、SOD和GSH-Px水平明显升高(t=6.562, t=1.971, t=1.972, P < 0.05);与模型组比较,地塞米松组、低和高剂量沙参麦冬汤组大鼠肺组织中MDA水平不同程度降低,且以高剂量沙参麦冬汤组效果最佳(P < 0.01)。见表 3。
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | Dose | MDA [mB/(μmol·g-1)] | SOD [λB/(U·g-1)] | GSH-Px [λB/(U·g-1×103)] | |||||||||||||||||||||||||
| Control | - | 23.36±7.21 | 62.01±8.90 | 4.01±0.39 | |||||||||||||||||||||||||
| Model | - | 76.22±4.20* | 83.12±6.69* | 5.52±0.69* | |||||||||||||||||||||||||
| Dexamethasone | 0.75 μg·kg-1 | 55.43±5.33△ | 77.88±7.65 | 5.08±0.23 | |||||||||||||||||||||||||
| Low dose of Radix Salviae Miltiorrhizae Decoction | 6.00 mg·kg-1 | 67.81±6.11 | 80.88±5.49 | 5.65±0.64 | |||||||||||||||||||||||||
| High dose of Radix Salviae Miltiorrhizae Decoction | 24.00 mg·kg-1 | 43.84±5.62△△ | 72.88±6.33△ | 4.78±0.33△ | |||||||||||||||||||||||||
| * P < 0.01 compared with control group; △ P < 0.05, △△ P < 0.01 compared with model group.“-”:No data. | |||||||||||||||||||||||||||||
与对照组比较,模型组大鼠肺组织中IL-1和TNF-α水平明显升高(t=20.241, t=32.808, P < 0.05);与模型组比较,地塞米松组、低和高剂量沙参麦冬汤组大鼠肺组织中IL-1及TNF-α水平不同程度降低(P < 0.05或P < 0.01),且以高剂量沙参麦冬汤组效果最佳。与对照组比较,模型组大鼠肺组织中IL-10水平明显降低(t=7.857, P < 0.05);与模型组比较,地塞米松组、低和高剂量沙参麦冬汤组大鼠肺组织中IL-10水平升高(P < 0.05或P < 0.01),且以高剂量沙参麦冬汤组效果最佳。与模型组比较,地塞米松组和沙参麦冬汤组大鼠肺组织中IL-1及TNF-α水平降低(P < 0.05或P < 0.01),IL-10水平升高(P < 0.05或P < 0.01)。见表 4。
| [n=10, x±s, ρB/(ng·L-1)] | |||||||||||||||||||||||||||||
| Group | Dose | IL-1 | TNF-α | IL-10 | |||||||||||||||||||||||||
| Control | - | 43.36±7.21 | 52.01±8.90 | 15.01±0.54 | |||||||||||||||||||||||||
| Model | - | 686.22±31.50* | 953.12±26.69* | 7.12±0.89* | |||||||||||||||||||||||||
| Dexamethasone | 0.75 μg·kg-1 | 476.22±30.20△△ | 676.22±37.20△△ | 10.22±0.46△ | |||||||||||||||||||||||||
| Low dose of Radix Salviae Miltiorrhizae Decoction | 6.00 mg·kg-1 | 553.84±28.61△ | 733.84±21.66△△ | 8.84±0.62△ | |||||||||||||||||||||||||
| High dose of Radix Salviae Miltiorrhizae Decoction | 24.00 mg·kg-1 | 313.84±27.62△△ | 402.88±21.33△△ | 12.88±0.33△△ | |||||||||||||||||||||||||
| * P < 0.01 compared with control group; △ P < 0.05, △△ P < 0.01 compared with model group.“-”:No data. | |||||||||||||||||||||||||||||
与对照组比较,模型组大鼠肺组织中Bax和caspase-3蛋白表达水平明显升高(t=22.663, t=19.234, P < 0.05);与模型组比较,地塞米松组、低和高剂量沙参麦冬汤组大鼠肺组织中Bax和caspase-3蛋白表达水平不同程度降低(P < 0.05或P < 0.01),且以高剂量沙参麦冬汤组效果最佳。与对照组比较,模型组大鼠肺组织中Bcl-2蛋白表达水平明显降低(t=7.743, P < 0.05);与模型组比较,地塞米松组、低和高剂量沙参麦冬汤大鼠肺组织中Bcl-2蛋白表达水平升高(P < 0.05或P < 0.01),且以高剂量沙参麦冬汤组效果最佳。见图 2和表 5。
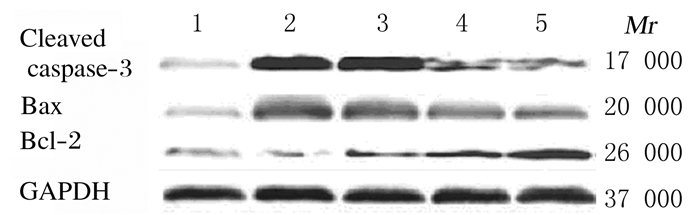
|
| Lane 1: Control group; Lane 2: Model group; Lane 3: Dexamethasone group; Lane 4: Low dose of Radix Salviae Miltiorrhizae Decoction group; Lane 5: High dose of Radix Salviae Miltiorrhizae Decoction group. 图 2 各组小鼠肺组织中Bax、Bcl-2和caspase-3蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of Bax, Bcl-2, and caspase-3 proteins in lung tissue of rats in various groups |
|
|
| (n=10, x±s) | |||||||||||||||||||||||||||||
| Group | Dose | Caspase-3 | Bax | Bcl-2 | |||||||||||||||||||||||||
| Control | - | 0.280±0.030 | 0.120±0.026 | 0.750±0.047 | |||||||||||||||||||||||||
| Model | - | 1.220±0.041* | 0.890±0.024* | 0.330±0.031* | |||||||||||||||||||||||||
| Dexamethasone | 0.75 μg·kg-1 | 0.710±0.038△ | 0.570±0.084△ | 0.620±0.042△△ | |||||||||||||||||||||||||
| Low dose of Radix Salviae Miltiorrhizae Decoction | 6.00 mg·kg-1 | 1.010±0.077 | 0.760±0.035△ | 0.480±0.029△ | |||||||||||||||||||||||||
| High dose of Radix Salviae Miltiorrhizae Decoction | 24.00 mg·kg-1 | 0.590±0.044△△ | 0.480±0.025△△ | 0.700±0.062△△ | |||||||||||||||||||||||||
| * P < 0.01 compared with control group; △ P < 0.05, △△ P < 0.01 compared with model group. | |||||||||||||||||||||||||||||
研究[12-13]证实:早产儿BPD的发生与肺部氧化应激有关,极低出生质量婴儿置于高氧环境中可能会导致BPD。另有研究[14-15]表明:机械通气造成的高气压、高容量也会直接损伤气道和肺泡上皮细胞,使肺结构遭到破坏,发生肺泡融合和肺气肿,肺泡渗出加重,当肺修复时,易发生纤维化, 最终导致BPD的发生。本研究参考该原理,采用高氧吸入的方法,建立新生鼠支气管肺发育不良模型。当出现氧中毒时,大鼠肺泡上皮细胞和毛细血管内皮细胞可能受到直接损伤,使肺泡毛细血管通透性增加。动物实验[16]表明:新生小鼠暴露于高氧的强度决定了发育中气道结构和功能的改变。而早产儿暴露于高强度氧时,未能及时清除体内的氧自由基而导致细胞中出现严重的氧化应激反应,造成亲氧化剂和抗氧化剂失衡[17]。当组织抗氧化防御系统超负荷就会造成氧中毒,此时会发生一系列的炎症反应,中性粒细胞和巨噬细胞募集并被激活,导致了肺损伤。此外,不论在正常或是受损的肺组织中,机械通气带来的高气压和高潮气量均可导致毛细血管内皮细胞、肺泡上皮细胞及基底膜破裂, 同样会引起肺损伤,而该效应引起的细胞因子的快速产生,最终导致炎症因子与抗炎因子分泌失衡,引起炎症瀑布级联反应, 加重了肺组织的损伤[18-19]。
研究[20]显示:早产儿BPD与机械通气密切相关,亦是诱发早产儿发生BPD的独立因素,气道压力和吸入氧浓度随着机械通气时间延长而升高,BPD的发生率也随之升高。模型组大鼠氧暴露21d后肺泡腔扩大、结构简化,间隔增厚,RAC明显减少,炎症细胞浸润。模型组大鼠肺组织W/D值明显升高,可见明显肺水肿,模型组大鼠肺组织中MDA、SOD和GSH-Px水平明显升高,促炎因子IL-1和TNF-α水平明显升高,而抑炎因子IL-10水平明显降低,Bax及caspase-3蛋白表达水平明显升高,与BPD的病理特征相符。研究[7-8]显示:产前应用糖皮质激素可加速胎儿肺成熟, 促使胎儿肺间质变薄,从而改善早产儿的肺功能,早产儿应用糖皮质激素可减轻肺水肿和炎症反应,并减少对氧的依赖程度,但由于早产儿对糖皮质激素的反应不一,且不良反应较多,使得糖皮质激素的作用存在争议。中药的低毒和标本兼治的效果使其在临床上治疗早产儿肺发育不良越来越受到重视,本研究所用沙参麦冬汤由北沙参、玉竹、麦冬、天花粉、扁豆、桑叶、生甘草和地骨皮组成。方中北沙参具有免疫抑制作用,临床用其治疗慢性支气管炎。麦冬主要含皂苷、生物碱、谷甾醇、葡萄糖、氨基酸和维生素等,具有抗疲劳及清除自由基等作用[21-22]。与北沙参合用增强补肺阴的功效,而方中增加了桑叶等组分,增强了该方抗炎、清除自由基及减轻细胞损伤的作用。不同剂量沙参麦冬汤治疗均能明显逆转高氧造成的大鼠肺组织病理变化,减轻炎症因子的分泌,且大鼠肺水肿程度亦明显减轻。因此沙参麦冬汤加减能有效保护高氧造成的新生大鼠支气管及肺的损伤,并减少细胞凋亡,改善细胞发育不良的程度。
| [1] |
GHANTA S, LEEMAN KT, CHRISTOU H. An update on pharmacologic approaches to bronchopulmonary dysplasia[J]. Semin Perinatol, 2013, 37(2): 115-123. DOI:10.1053/j.semperi.2013.01.008 |
| [2] |
FERNÁNDEZ R, D'APREMONT I, DOMÍNGUEZ A, et al. Survival and morbidity of very low birth weight infant in a South American neonatal network[J]. Arch Argent Pediatr, 2014, 112(5): 405-412. |
| [3] |
SHAH PS, LUI K, SJÖRS G, et al. Neonatal outcomes of very low birth weight and very preterm neonates: An international comparison[J]. J Pediatr, 2016, 177: 144-152. DOI:10.1016/j.jpeds.2016.04.083 |
| [4] |
RICE MS, VALENTINE CJ. Neonatal body composition: measuring lean mass as a tool to Guide nutrition management in the neonate[J]. Nutr Clin Pract, 2015, 30(5): 625-632. DOI:10.1177/0884533615578917 |
| [5] |
PAPADOPOULOS D, MISTHOS P, CHORTI M, et al. Unilateral pulmonary hypoplasia in an adult patient[J]. Monaldi Arch Chest Dis, 2018, 88(1): 829. |
| [6] |
WANG T, MENG M, HUANG M, et al. Variations of right bronchial tree: A study with multi-detector CT[J]. Surg Radiol Anat, 2018, 40(3): 955-958. |
| [7] |
GLASBERG T, JACKSON P, PAVLOVA Z, et al. Infant with clinical evidence of pulmonary hypoplasia: A case report[J]. Cureus, 2017, 9(5): e1298. |
| [8] |
武慧, 韩彤妍, 王新利, 等. 超低/极低出生体重儿支气管肺发育不良的危险因素[J]. 中华围产医学杂志, 2016, 19(10): 761-765. |
| [9] |
RUBIN L P. Pulmonary hypoplasia resulting from prolonged rupture of membranes: A distinct clinical entity with instructive experimental models[J]. Pediatr Pulmonol, 2017, 52(11): 1378-1380. DOI:10.1002/ppul.23764 |
| [10] |
顾廉洁, 王珊珊, 郭敏敏, 等. 维生素A与维生素D在新生儿支气管肺发育不良防治中的研究进展[J]. 广西医学, 2018, 40(6): 681-684. |
| [11] |
MATURU P, WEI-LIANG Y H, ANDROUTSOPOULOS V P, et al. Quercetin attenuates the hyperoxic lung injury in neonatal mice: Implications for bronchopulmonary dysplasia (BPD)[J]. Food Chem Toxicol, 2018, 114: 23-33. DOI:10.1016/j.fct.2018.02.026 |
| [12] |
CORREIA L L, JOHNSON J A, MCERLEAN P, et al. SOX2 drives bronchial dysplasia in a novel organotypic model of early human squamous lung cancer[J]. Am J Respir Crit Care Med, 2017, 195(11): 1494-1508. DOI:10.1164/rccm.201510-2084OC |
| [13] |
BUSH D, ABMAN S H, GALAMBOS C. Prominent intrapulmonary bronchopulmonary anastomoses and abnormal lung development in infants and children with down syndrome[J]. J Pediatr, 2017, 180: 156-162. DOI:10.1016/j.jpeds.2016.08.063 |
| [14] |
NAKANISHI H, UCHIYAMA A, KUSUDA S. Impact of pulmonary hypertension on neurodevelopmental outcome in preterm infants with bronchopulmonary dysplasia: A cohort study[J]. J Perinatol, 2016, 36(10): 890-896. DOI:10.1038/jp.2016.108 |
| [15] |
MESTAN K K, GOTTEINER N, PORTA N, et al. Cord blood biomarkers of placental maternal vascular underperfusion predict bronchopulmonary dysplasia-as-sociated pulmonary hypertension[J]. J Pediatr, 2017, 185: 33-41. DOI:10.1016/j.jpeds.2017.01.015 |
| [16] |
BERGER J, BHANDARI V. Animal models of bronchopulmonary dysplasia.The term mouse models[J]. Am J Physiol Lung Cell Mol Physiol, 2014, 15, 307(12): L936-L947. |
| [17] |
早产儿支气管肺发育不良调查协作组. 早产儿支气管肺发育不良发生率及高危因素的多中心回顾调查分析[J]. 中华儿科杂志, 2011, 49(9): 655-662. |
| [18] |
PAN J R, ZHAN C Y, YUAN T M, et al. Effects and molecular mechanisms of intrauterine infection/inflammation on lung development[J]. Resp Res, 2018, 19(1): 93. DOI:10.1186/s12931-018-0787-y |
| [19] |
LIU Y, HOYO C, MURPHY S, et al. DNA methylation at imprint regulatory regions in preterm birth and infection[J]. Am J Obstet Gynecol, 2013, 208(5): 395. |
| [20] |
THOMAS W, SPEER C P. Chorioamnionitis is essential in the evolution of bronchopulmonary dysplasia:the case in favour[J]. Paediatr Respir Rev, 2014, 15(1): 49-52. |
| [21] |
洪素兰, 陈玉龙, 邵雷, 等. 沙参麦冬汤对肺阴虚型慢性支气管炎模型大鼠SIgA与IL-1、IL-6、TNF-α的影响[J]. 中国中医基础医学杂志, 2009, 15(12): 948. |
| [22] |
韩彦华. 沙参麦冬汤在小儿肺炎恢复期中的应用[J]. 临床合理用药, 2009, 2(24): 49. |
 2019, Vol. 45
2019, Vol. 45


