扩展功能
文章信息
- 周宁, 郭纪伟, 代娟娟, 李雪琳, 席思川, 宫凯凯, 武艳
- ZHOU Ning, GUO Jiwei, DAI Juanjuan, LI Xuelin, XI Sichuan, GONG Kaikai, WU Yan
- YAP对非小细胞肺癌细胞增殖和迁移能力的影响
- Effect of YAP on proliferation and migration of non-small cell lung cancer cells
- 吉林大学学报(医学版), 2019, 45(06): 1320-1326
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1320-1326
- 10.13481/j.1671-587x.20190622
-
文章历史
- 收稿日期: 2019-01-17
2. 滨州医学院附属医院肿瘤研究中心, 山东 滨州 256600
2. Cancer Research Center, Affiliated Hospital, Binzhou Medical University, Binzhou 256603, China
肺癌是最常见的恶性肿瘤之一,位居癌症相关死亡的第一位,严重危害人类的健康。肺癌分为小细胞肺癌(small cell lung cancer,SCLC)和非小细胞肺癌(non-small cell lung cancer,NSCLC),其中NSCLC占85%以上[1]。YAP蛋白是MST信号通路中的关键组分之一,在哺乳类动物中高度保守,在调控细胞增殖和器官发育中发挥重要作用[2]。MST1/2-LATS1/2的级联激酶反应通过磷酸化反应下调YAP的表达量[3]。活化的YAP能够进入细胞核,通过与TEAD家族相互作用促进survivin等抗凋亡基因的表达[4]。YAP异常表达能够促进细胞的恶性转化和转移,并提高其耐药能力[5]。近年研究[6-8]显示:YAP在肝癌、肺癌、乳腺癌、结肠癌和卵巢癌等多种人类肿瘤细胞中呈高表达状态,并与肿瘤的发生发展和转移有密切关系。MALAT1是第1个被确定的与肺癌密切相关的长链非编码RNA(long non-coding RNA, lncRNA),现已证实其在多种肿瘤中表达上调,并与肿瘤的迁移浸润密切相关[9]。在人类SK-N-SH成神经细胞瘤中,催产素通过cAMP应答元件与MALAT1启动子的转录因子结合,上调MALAT1的表达[10], 在能够表达雌激素受体α(estrogen receptor-α,ER-α)的乳腺癌组织中,17β-雌二醇(17β-estradiol)能够下调MALAT1的转录,进而抑制细胞增殖、迁移和浸润[11]。已有研究[12]显示:在肝癌组织中YAP能够上调MALAT1的表达水平,但在NSCLC组织中,YAP与MALAT1的调控关系及MALAT1在YAP介导的细胞增殖和迁移过程中的作用尚未见报道。本研究通过基因修饰和分子生物学手段研究NSCLC中YAP对MALAT1的调控作用及MALAT1在YAP介导的NSCLC细胞增殖和迁移中的作用。
1 材料与方法 1.1 细胞、主要试剂和仪器A549和H1299细胞系购于美国ATCC细胞库。胎牛血清、RPMI-1640培养基和胰酶购自美国Gibco公司,Lipofectamine 2000购于美国Invitrogen公司, siRNA由上海吉玛生物公司合成,引物购于上海生物工程有限公司,逆转录试剂盒和RT-PCR试剂盒购于南京Vazyme公司,pcDNA3.1和pcDNA3.1-YAP载体均为本实验室保存,YAP单克隆抗体购于美国Abcam公司,Tubulin抗体和辣根过氧化物酶标记的羊抗兔IgG抗体购于美国Proteintech公司,CCK-8试剂购于上海碧云天公司。CO2细胞培养箱购于美国Thermo公司,正置显微镜购于德国Leica公司,酶标仪购于美国BioTek公司。
1.2 细胞培养A549和H1299细胞采用含有10%胎牛血清和1%青霉素-链霉素的RPMI1640培养基,置于37 ℃、饱和湿度、5%CO2的培养箱中培养。
1.3 细胞转染将待转染的质粒或siRNA与Lipofectamine 2000混合后,加入100 μL不含FBS的RIPM1640培养基中,轻轻混匀,室温静置25 min后,加入到密度长至60 % ~ 80 %的A549和H1299细胞中,将2种细胞分别分为Scramble siRNA组(转染Scramble siRNA)、siYAP-1组(转染siYAP-1)和siYAP-2组(转染siYAP-2)或Scramble siRNA组(转染Scramble siRNA)、siMALAT1-1(转染siMALAT1-1)和siMALAT1-2组(转染siMALAT1-2)。继续培养48h, 进行相关实验。
1.4 RT-PCR法检测各组细胞中YAP和MALAT1mRNA表达水平收集转染48 h后的A549和H1299细胞,采用TRIzol法提取细胞总RNA,并用逆转录试剂盒合成cDNA,采用AceQ Universal SYBR qPCR Master Mix进行PCR检测,以GAPDH作为内参,进行3次重复实验,采用2-ΔΔCT法计算YAP和MALAT1 mRNA表达水平。PCR引物:YAP正向引物,5′- CGCTCTTCAACGCCGTCA-3′,YAP反向引物,5′- AGTACTGGCCTGTCGGGAGT-3′;MALAT1正向引物,5′-GCGACGAGTTGTGCTGCTATCT-3′,MALAT1反向引物,5′-ACACTGCTCTGGGTCTGCTTTT-3′; GAPDH正向引物,5′-CTCCTCCTGTTCGACAGTCAGC-3′,GAPDH反向引物,5′-CCCAATACGACCAAATCCGTT-3′。siRNA抑制效率=(ScramblesiRNA组mRNA相对表达水平-siYAP组或siMALAT1组mRNA相对表达水平)/Scramble siRNA组mRNA相对表达水平×100%。
1.5 Western blotting检测各组细胞中YAP蛋白表达水平收集转染48 h后各组A549或H1299细胞,NP-40裂解液裂解细胞并通过BCA法测定蛋白浓度。取30 μg蛋白上样,进行SDS-PAGE电泳;转膜、封闭后分别孵育anti-YAP(1:1 000)和anti-Tubulin(1:1 000)抗体过夜,次日孵育HRP标记的羊抗兔IgG抗体(1 :1 000),通过ECL底物显色,最后应用成像系统进行成像拍照,实验重复3次,采用ImageJ分析软件,以Tubulin蛋白为内参,计算目的蛋白表达水平。目的蛋白表达水平=目的蛋白灰度值/内参蛋白灰度值。
1.6 CCK-8法检测各组细胞增殖能力收集转染pcDNA3.1-YAP质粒(pcDNA3.1-YAP组)、共转染pcDNA3.1-YAP质粒和siMALAT1-2(pcDNA3.1-YAP+ siMALAT1-2组)及其对照质粒(pcDNA3.1-YAP+Scramble siRNA组)的A549或H1299细胞,采用台酚蓝计数,将细胞以每孔5×103个的密度接种于96孔板,每孔接种100 μL细胞悬液,设置3个平行孔,培养48 h后,每孔加入10 μL CCK-8溶液,继续培养2 h,应用酶标仪测定各孔在450 nm波长处的吸光度(A)值,实验重复3次,取平均A值,以A值代表细胞的增殖能力,对对照组进行均一化处理后,采用GraphPad Prism 7.0软件绘制细胞增殖能力图。
1.7 划痕实验检测细胞迁移能力收集转染pcDNA3.1-YAP(pcDNA3.1-YAP组)、共转染pcDNA3.1-YAP和siMALAT1-2(pcDNA3.1-YAP+ siMALAT1-2组)及其对照Scramble siRNA(pcDNA3.1-YAP+Scramble siRNA组)的H1299细胞,采用200 μL枪头进行划痕,PBS洗涤后加入无血清的培养基继续培养48 h,并分别在划痕0、24和48 h时进行拍照,实验重复3次,采用Image J软件对划痕距离进行分析,并应用Graph Pad Prism 7.0软件对各组细胞划痕愈合率绘制图表。细胞划痕愈合率=(0 h划痕间距- 24或48 h划痕间距)/0 h划痕间距×100%。
1.8 统计学分析采用SPSS 20.0统计软件进行统计学分析。各组细胞中YAP和MALAT1 mRNA表达水平及YAP蛋白表达水平、细胞增殖能力、细胞划痕愈合率均以x±s表示,多组间样本均数比较采用单因素方差分析,组间均数多重比较采用LSD-t检验。以P < 0.05为差异有统计学意义。
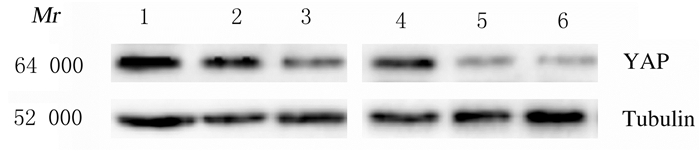
2 结果 2.1 各组细胞中YAPmRNA和蛋白及MALAT1mRNA表达水平siRNA转染后,与Scramble siRNA组比较,siYAP-1和siYAP-2组A549和H1299细胞中YAP mRNA表达水平均明显降低(A549:t=0.32,P < 0.05;t=0.55,P < 0.05;H1299:t=0.295,P < 0.05;t=0.47,P < 0.05),2种siRNA在A549细胞中对YAP mRNA表达的抑制效率分别为(35.0±2.1)%和(55.2±3.9)%,在H1299细胞分别为(29.5±2.1)%和(47.1±4.2)%。见图 1。Western blotting法检测结果显示:siYAP-1和siYAP-2组A549和H1299细胞中YAP蛋白表达水平明显降低。见图 2。RT-PCR法检测结果显示:siYAP-1和siYAP-2组A549和H1299细胞中MALAT1mRNA表达水平低于Scramble siRNA组(A549:t=0.3900,P < 0.05;t=0.5350,P < 0.05;H1299:t=0.3515,P < 0.05;t=0.4150,P < 0.05)。见图 3。

|
| A:A549 cells; B:H1299 cells.*P < 0.05, **P < 0.01 vs Scramble siRNA group. 图 1 RT-PCR法检测各组细胞中YAP mRNA表达水平 Fig. 1 Expression levels of YAP mRNA in cells in various groups detected by RT-PCR method |
|
|
|
| Lane 1, 4: Scramble siRNA group; Lane 2, 5: siYAP-1 group; Lane 3, 6: siYAP-2 group; Lane 1-3:A459 cells; Lane 4-6:H1299 cells. 图 2 Western blotting法检测各组细胞中YAP蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of YAP protein in cells in various groups detected by Western blotting method |
|
|
|
| A:A549 cells; B:H1299 cells.*P < 0.05, **P < 0.01 vs Scramble siRNA group. 图 3 RT-PCR法检测各组细胞中MALAT1 mRNA表达水平 Fig. 3 Expression levels of MALAT1 mRNA in cells in various groups detected by RT-PCR method |
|
|
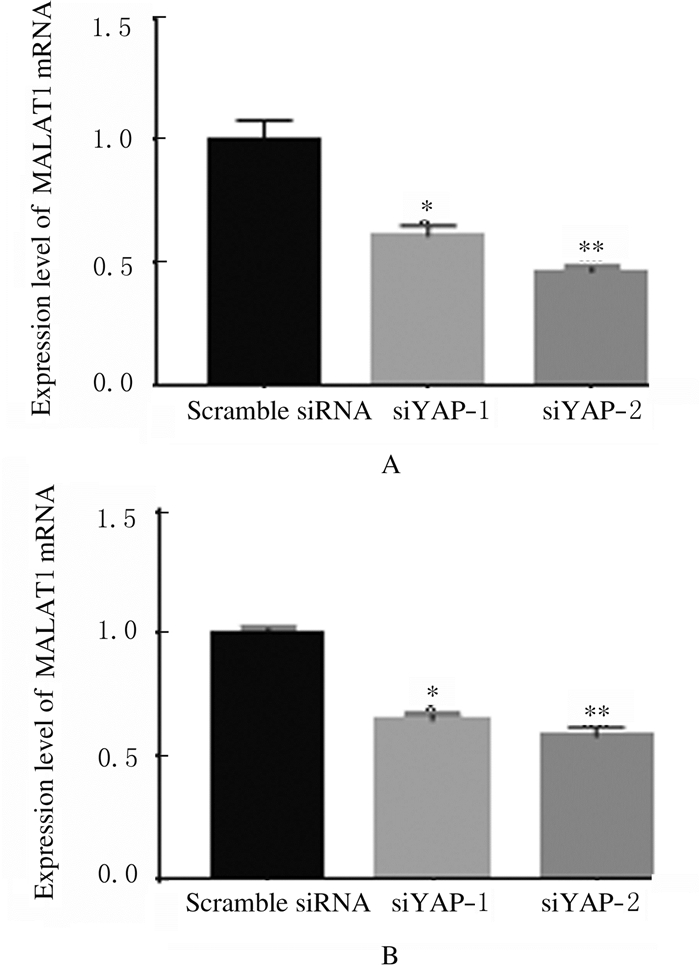
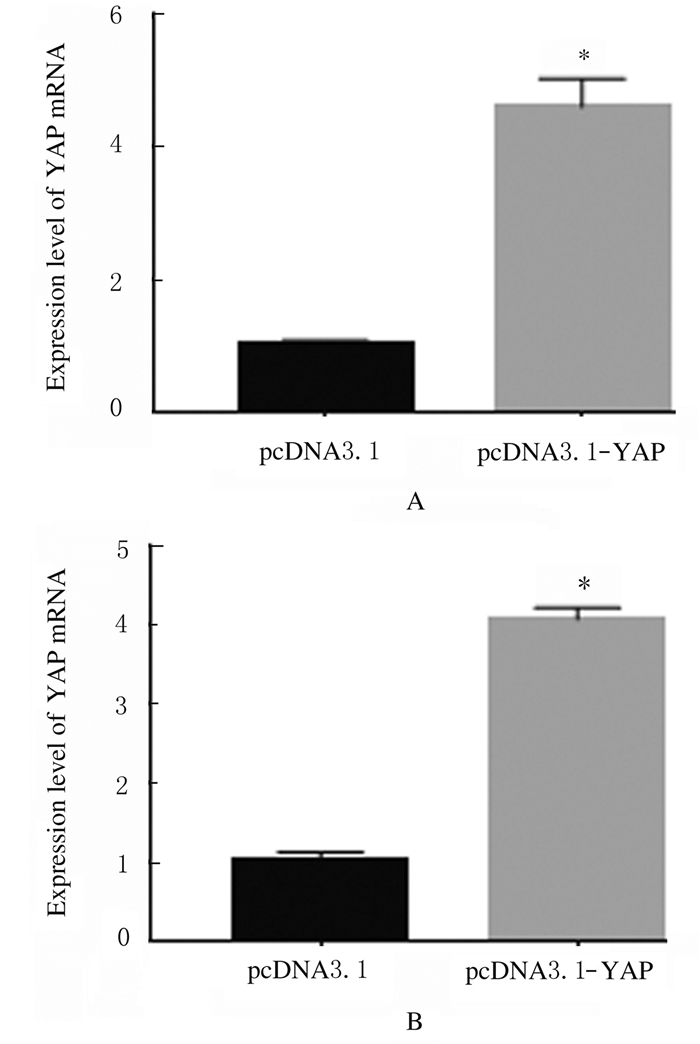
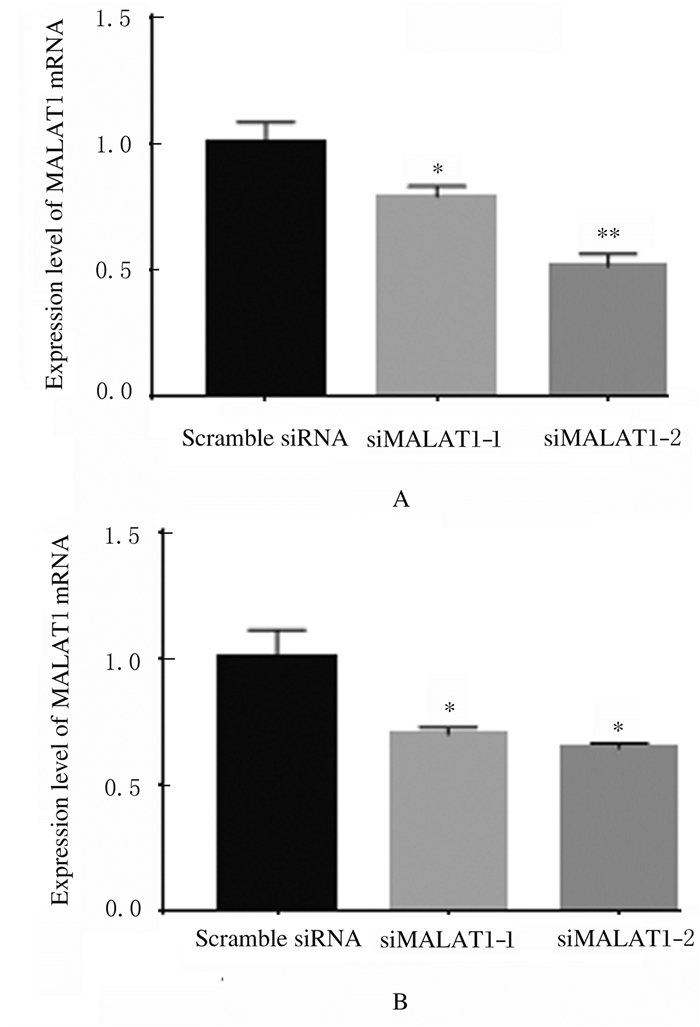
转染pcDNA3.1-YAP后,A549和H1299细胞中YAP mRNA表达水平较pcDNA3.1组分别提高了4.5倍和4.0倍(P < 0.05),见图 4。siMALAT1-1和siMALAT1-2组A549和H1299细胞中MALAT1 mRNA表达水平明显降低(A549:t=0.210,P < 0.05;t=0.485,P < 0.05,H1299:t=0.300,P < 0.05;t=0.355,P < 0.05),A549细胞中siRNA对MALAT1mRNA表达的抑制效率分别为(21.0±4.2)%和(48.5±4.9)%,在H1299细胞中分别为(30.1±2.8)%和(35.5±2.1)%。见图 5。可见siMALAT1-2的作用效果更好,因此选用siMALAT1-2进行后续实验。
|
| *P < 0.01 vs pcDNA3.1 group; A:A549 cells; B:H1299 cells. 图 4 RT-PCR法检测转染pcDNA3.1前后细胞中YAP mRNA表达水平 Fig. 4 Expression levels of YAP mRNA in cells before and after transfected with pcDNA3.1 detected by RT-PCR method |
|
|
|
| A:A549 cells; B:H1299 cells.*P < 0.05, **P < 0.01 vs Scramble siRNA group. 图 5 RT-PCR法检测转染siMALAT1后细胞中MALAT1 mRNA表达水平 Fig. 5 Expression levels of MALAT1 mRNA in cells after transfected with siMALAT1 detected by RT-PCR method |
|
|
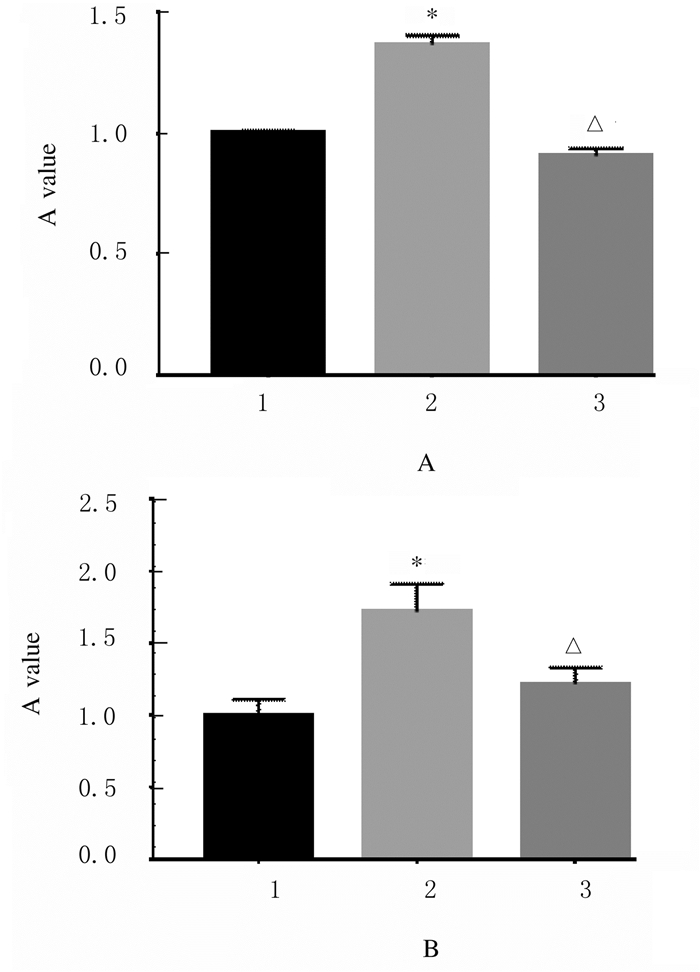
CCK-8实验结果表明: pcDNA3.1-YAP+ scramble siRNA组A549和H1299细胞的增殖能力(1.00±0.02, 1.00±0.11)低于pcDNA3.1-YAP组(1.36±0.04, 1.71±0.19)(A549:t=-0.3674,P < 0.05;H1299:t=-0.7157,P < 0.05),表明过表达YAP能显著促进A549和H1299细胞的增殖(图 6);pcDNA3.1-YAP+siMALAT1-2组A549和H1299细胞的相对增殖能力分别为(0.91±0.03,1.20±0.11),低于pcDNA3.1-YAP组(A549:t=-0.4623,P < 0.05;H1299:t =-0.499,P < 0.05)。
|
| 1:pcDNA3.1-YAP group; 2:pcDNA3.1-YAP+Scramble siRNA group; 3:pcDNA3.1-YAP+siMALAT1-2 group.*P < 0.01 vs pcDNA3.1-YAP+Scramble siRNA group; △P < 0.05 vs pcDNA3.1-YAP group. 图 6 CCK-8法检测各组A549(A)和H1299(B)细胞的增殖能力 Fig. 6 Proliferation abilities of A549 (A) and H1299 (B) cells in various groups detected by CCK-8 method |
|
|
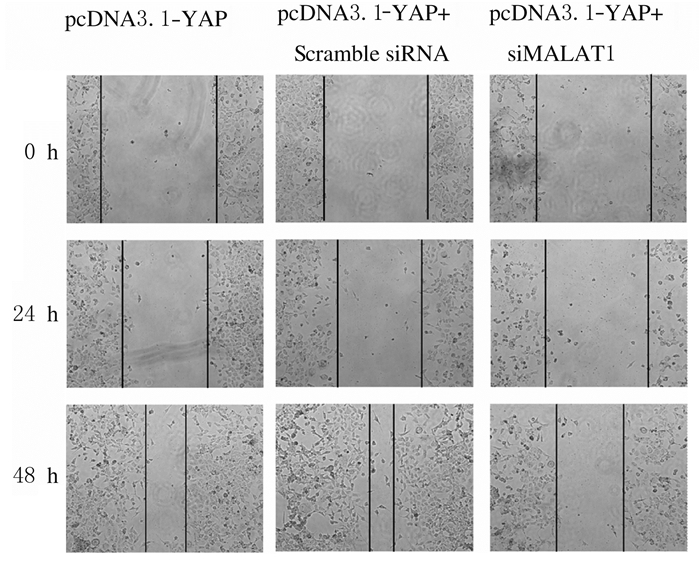
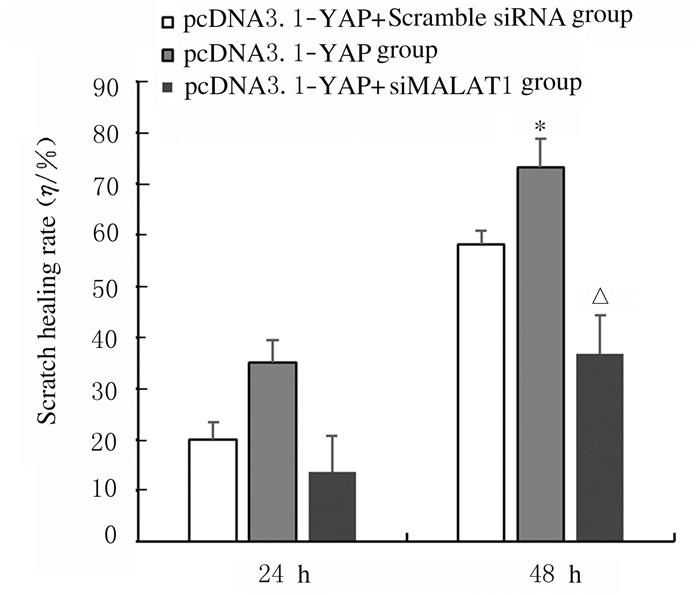
划痕实验结果表明: 48 h时pcDNA3.1-YAP组H1299细胞划痕愈合率为(73.20±5.52)%,明显高于pcDNA3.1-YAP+ Scramble siRNA组(58.20%±2.58%),差异有统计学意义(t=12.8,P < 0.05),48 h时pcDNA3.1-YAP+siMALAT1-2组H1299细胞划痕愈合率为(36.70%±7.58%), 明显低于pcDNA3.1-YAP组(t=-36.54,P < 0.05)。见图 7和8。
|
| 图 7 划痕实验检测各组H1299细胞迁移能力 Fig. 7 Migration abilities of H1299 cells in various groups detected by scratch assay |
|
|
|
| *P < 0.05 vs pcDNA3.1-YAP+Scramble siRNA group; △P < 0.05 vs pcDNA3.1-YAP group. 图 8 各组H1299细胞划痕愈合率 Fig. 8 Scratch healing rates of H1299 cells in various groups |
|
|
近年来,肺癌尤其是NSCLC的患病率和死亡率呈逐年升高的趋势。由于诊断方法和外科技术等的不断发展,NSCLC患者的寿命在很大程度上得以延长,但是由于缺少有效的靶向治疗,5年内多数患者均会出现疾病复发的现象。因此,寻求有效和特异性治疗NSCLC的靶点至关重要。
研究[13-14]显示:YAP信号通路在调控肿瘤的增殖和迁移等发面发挥重要作用,但YAP下游调控机制尚不十分完善。本课题组前期研究[15]显示:去甲斑蝥素可通过抑制YAP的表达抑制NSCLC细胞增殖、迁移、浸润和上皮细胞-间充质转化(epithelial-mesenchymal transformation, EMT)转化。MALAT1通过与SR蛋白家族[16]、DGCR8[17]和AGO2[18]等相互作用,与RNA剪切、NSCLC患者的转移[19-20]密切相关,并能影响一系列参与细胞能动性基因的表达[21]。已有研究[12]表明:在肝癌组织中YAP能够在转录水平及转录后水平上调MALAT1的表达,但该机制在NSCLC中尚不明确。本文作者根据文献及前期实验[15]结果推测:NSCLC中YAP可能通过调控MALAT1的表达来影响NSCLC细胞的增殖和迁移能力。本研究采用RNA干扰技术(iRNA),在A549和H1299细胞转染靶向YAP的siRNA后发现细胞状态未见明显异常。本研究结果显示:siYAP-1和siYAP-2组A549和H1299细胞中YAP mRNA和蛋白表达水平较Scramble siRNA组明显下调,说明siYAP-1和siYAP-2能够准确高效抑制靶基因表达;与Scramble siRNA组比较,沉默YAP基因后siYAP-1和siYAP-2组细胞中MALAT1mRNA表达明显受到抑制;为确定MALAT1在YAP介导的NSCLC细胞增殖和迁移中的作用,本课题组首先验证真核载体pcDNA3.1-YAP的表达情况和靶向MALAT1的siRNA的抑制效率,结果显示:与Scramble siRNA组比较,siYAP-1和siYAP-2组A549和H1299细胞中YAP mRNA表达水平明显升高;CCK-8法和划痕实验检测结果显示:pcDNA3.1-YAP+Scramble siRNA组A549和H1299细胞的增殖能力和迁移能力均明显低于pcDNA3.1-YAP组,并且pcDNA3.1-YAP组细胞的增殖能力和迁移能力均明显高于pcDNA3.1-YAP+siMALAT1组,表明MALAT1参与了YAP介导的NSCLC细胞的增殖和迁移过程。YAP可以与细胞核内转录激活因子TEAD家族蛋白结合,调控肿瘤细胞内多种癌基因的转录,推测YAP可能通过激活MALAT1基因的转录,促进其表达,并且MALAT1可能也参与了YAP介导的细胞迁移[22]、转化[23]和肿瘤形成[24]等过程,但该推测还有待进一步研究。
综上所述,YAP可以调控NSCLC细胞中MALAT1的表达,并且MALAT1在YAP介导的细胞增殖和迁移中发挥重要作用。因此,继续研究YAP调控MALAT1表达的机制,对完善肺癌中YAP的调控网络及研究MALAT1在NSCLC中的作用非常必要。
| [1] |
HERBST R S, MORGENSZTERN D, BOSHOFF C. The biology and management of non-small cell lung cancer[J]. Nature, 2018, 553(7689): 446-454. DOI:10.1038/nature25183 |
| [2] |
WEI T, WEILER S, TÓTH M, et al. YAP-dependent induction of UHMK1 supports nuclear enrichment of the oncogene MYBL2 and proliferation in liver cancer cells[J]. Oncogene, 2019, 38(27): 5541-5550. DOI:10.1038/s41388-019-0801-y |
| [3] |
ZHAO B, LI L, LEI Q Y, et al. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version[J]. Genes Dev, 2010, 24(9): 862-874. DOI:10.1101/gad.1909210 |
| [4] |
HAO Y W, CHUN A, CHEUNG K, et al. Tumor suppressor LATS1 is a negative regulator of oncogene YAP[J]. J Biol Chem, 2008, 283(9): 5496-5509. DOI:10.1074/jbc.M709037200 |
| [5] |
LAI C J, LIN C Y, LIAO W Y, et al. CD44 promotes migration and invasion of docetaxel-resistant prostate cancer cells likely via induction of hippo-Yap signaling[J]. Cells, 2019, 8(4): E295. DOI:10.3390/cells8040295 |
| [6] |
MOROOSHI T, HAYASHI T, PAN W W, et al. The hippo pathway kinases LATS1/2 suppress cancer immunity[J]. Cell, 2016, 167(6): 1525-1539. DOI:10.1016/j.cell.2016.11.005 |
| [7] |
LO S F, STRANO S, BLANDINO G. YAP and TAZ in lung cancer: oncogenic role and clinical targeting[J]. Cancers (Basel), 2018, 10(5): E137. DOI:10.3390/cancers10050137 |
| [8] |
WANG E Y, CHENG J C, THAKUR A, et al. YAP transcriptionally regulates ErbB2 to promote liver cell proliferation[J]. Biochim Biophys Acta, 2018, 1861(9): 854-863. DOI:10.1016/j.bbagrm.2018.07.004 |
| [9] |
ZHUANG M, ZHAO S L, JIANG Z, et al. MALAT1 sponges miR-106b-5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 enhanced microtubules mobility[J]. EBioMedicine, 2019, 41: 286-298. DOI:10.1016/j.ebiom.2018.12.049 |
| [10] |
MOROISHI T, HAYASHI T, Pan W W, et al. The hippo pathway kinases lats1/2 suppress cancer immunity[J]. Cell, 2016, 167(6): 1525-1539. DOI:10.1016/j.cell.2016.11.005 |
| [11] |
ZHAO Z, CHEN C, LIU Y, et al. 17β-Estradiol treatment inhibits breast cell proliferation, migration and invasion by decreasing MALAT-1 RNA level[J]. Biochem Biophys Res Commun, 2014, 445(2): 388-393. DOI:10.1016/j.bbrc.2014.02.006 |
| [12] |
WANG J Y, WANG H M, ZHANG Y, et al. Mutual inhibition between YAP and SRSF1 maintains long non-coding RNA, Malat1-induced tumourigenesis in liver cancer[J]. Cell Signal, 2014, 26(5): 1048-1059. DOI:10.1016/j.cellsig.2014.01.022 |
| [13] |
CHAN S W, LIM C J, CHONG Y F, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin[J]. J Biol Chem, 2011, 286(9): 7018-7026. DOI:10.1074/jbc.C110.212621 |
| [14] |
LIANG Z G, WANG Y Y, SHEN Z Y, et al. Fascin 1 promoted the growth and migration of non-small cell lung cancer cells by activating YAP/TEAD signaling[J]. Tumour Biol, 2016, 37(8): 10909-10915. DOI:10.1007/s13277-016-4934-0 |
| [15] |
GUO J W, WU Y, YANG L J, et al. Repression of YAP by NCTD disrupts NSCLC progression[J]. Oncotarget, 2017, 8(2): 2307-2319. |
| [16] |
TRIPATHI V, ELLIS J D, SHEN Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation[J]. Mol Cell, 2010, 39(6): 925-938. DOI:10.1016/j.molcel.2010.08.011 |
| [17] |
MACIAS S, PLASS M, STAJUDA A, et al. DGCR8 HITS-CLIP reveals novel functions for the microprocessor[J]. Nat Struct Mol Biol, 2012, 19(8): 760-766. DOI:10.1038/nsmb.2344 |
| [18] |
MIYAGAWA R, TANO K, MIZUNO R, et al. Identification of cis-and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles[J]. RNA, 2012, 18(4): 738-751. DOI:10.1261/rna.028639.111 |
| [19] |
JI P, DIEDERICHS S, WANG W B, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer[J]. Oncogene, 2003, 22(39): 8031-8041. DOI:10.1038/sj.onc.1206928 |
| [20] |
GUTSCHER T, HÄMMERLE M, EISSMANN M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells[J]. Cancer Res, 2013, 73(3): 1180-1189. DOI:10.1158/0008-5472.CAN-12-2850 |
| [21] |
TANO K, MIZUNO R, OKADA T, et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes[J]. FEBS Lett, 2010, 584(22): 4575-4580. DOI:10.1016/j.febslet.2010.10.008 |
| [22] |
ZHAO J, LI X N, YANG Y, et al. Effect of YAP1 silencing on esophageal cancer[J]. Onco Targets Ther, 2016, 9: 3137-3146. |
| [23] |
DONG L, LIN F, WU W J, et al. Transcriptional cofactor Mask2 is required for YAP-induced cell growth and migration in bladder cancer cell[J]. J Cancer, 2016, 7(14): 2132-2138. DOI:10.7150/jca.16438 |
| [24] |
ZANCONATO F, CORDENONSI M, PICCOLO S. YAP/TAZ at the roots of cancer[J]. Cancer Cell, 2016, 29(6): 783-803. DOI:10.1016/j.ccell.2016.05.005 |
 2019, Vol. 45
2019, Vol. 45


