扩展功能
文章信息
- 汤淼, 艾浩, 李晓明
- TANG Miao, AI Hao, LI Xiaoming
- RhoA和ROCK蛋白在高脂饮食幼鼠肾小球中的表达及其意义
- Expressions of RhoA and ROCK proteins in glomeruli of young mice fed with high-fat diet and their significances
- 吉林大学学报(医学版), 2019, 45(06): 1315-1319
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1315-1319
- 10.13481/j.1671-587x.20190621
-
文章历史
- 收稿日期: 2019-01-15
2. 辽宁省卵泡发育与生殖健康重点实验室, 辽宁 锦州 121001;
3. 广东药科大学组织学与胚胎学教研室, 广东 广州 510006
2. Key Laboratory of Follicular Development and Reproductive Health, Jinzhou Mediacal University, Jinzhou 121001, China;
3. Department of Histology and Embryology, Guangdong Pharmaceutical University, Guangzhou 510006, China
高脂饮食(high-fat diet,HFD)与肥胖和脂质代谢紊乱有关的疾病密切相关[1],而肥胖及脂质代谢紊乱可进一步诱发多种肾脏疾病[2]。Rho蛋白为小分子鸟嘌呤核苷酸结合蛋白,被称为小G蛋白,其中RAC1、Cdc42和RhoA在Rho家族蛋白中研究最为广泛,其在细胞极化、细胞与基质间黏附、细胞迁移、细胞增殖和凋亡中均发挥重要作用[3]。ROCK,为Rho激酶,是目前研究最广泛的Rho下游靶效应分子[4]。ROCK可接收Rho传递的信号,并介导下游一系列磷酸化/脱磷酸化反应,从而广泛参与细胞的黏附、运动、增殖、凋亡和基因表达等行为[5]。RhoA/ ROCK信号通路在慢性肾脏疾病进展中起重要作用,但目前关于RhoA/ROCK信号通路是否参与调控HFD诱导的肾脏损伤尚未明确。本研究通过HFD喂养C57BL/6J幼鼠12周,观察幼鼠肾组织病理形态表现和肾组织中RhoA及ROCK蛋白表达的变化,探讨RhoA/ROCK通路在HFD所致肾小球损伤中的作用及意义。
1 材料与方法 1.1 实验动物、主要试剂和仪器3周龄雄性C57BL/6J幼鼠36只,SPF级,购自北京维通利华实验动物技术有限公司,动物合格证号:SCXK(京)2016-0011。RhoA兔多克隆抗体、ROCK兔多克隆抗体和vimentin兔多克隆抗体购自美国Abcam公司,GAPDH鼠单克隆抗体、辣根过氧化物酶(HRP)标记的羊抗兔IgG、HRP标记的羊抗鼠IgG和PV二步法免疫组织化学检测试剂盒购自北京中杉金桥生物科技有限公司,PAS染色试剂盒和Masson三色染色试剂盒购自中国北京索莱宝科技有限公司,ECL显色试剂盒购自美国Thermo公司,高脂饲料(60 kcal% Fat Research Diets)和标准饲料(10 kcal% Fat Research Diets)购自美国Reserch Diets公司。Olympus AU400生化仪Olympus显微镜和照相机购自日本Olympus公司,Leica RF2235石蜡切片机购自日本Leica公司,电泳仪和转膜仪购自美国Bio-Rad公司。
1.2 动物分组和处理方法将幼鼠随机分为正常对照组和HFD组,每组18只。正常对照组幼鼠给予标准饲料,HFD组幼鼠给予高脂饲料。将2组3周龄幼鼠分别饲养4、8和12周后称质量。12周后取材,4%多聚甲醛行心脏灌流,左肾放入4%多聚甲醛固定液中固定,石蜡包埋,切5 μm厚度的石蜡切片,右肾放入-80℃冰箱保存用于免疫印迹检测。
1.3 2组幼鼠血清总胆固醇(total cholesterol, TC)和甘油三酯(triglyceride, TG)水平检测将2组幼鼠分别饲养12周后检测血清TC和TG水平。幼鼠麻醉后,采取眼眶静脉血,将血液室温下静置1 h,再离心取上清,采用Olympus AU400生化仪检测2组幼鼠血清TC和TG水平。
1.4 HE染色、PAS染色和Masson染色检测2组幼鼠肾组织病理形态表现、肾小球系膜和基底膜相对面积及肾间质纤维组织相对面积将5 μm石蜡切片脱蜡,行苏木精、伊红浸染,脱水,透明,封片。将5 μm切片脱蜡,2%高碘酸浸染15 min,schiff浸染1 h,苏木精复染,脱水,透明,封片。采用Imagepro Plus 6.0软件进行PAS染色分析,每张切片选3个视野,计算肾小球系膜和基底膜相对面积。肾小球系膜和基底膜相对面积=PAS阳性染色面积/肾小球毛细血管袢总面积×100%[6]。将5 μm切片脱蜡,苏木素浸染2 min,丽春红酸性品红液浸染5 min,醋酸苯胺蓝复染5 min,脱水,透明,封片。采用Imagepro Plus 6.0软件进行Masson染色分析,每张切片选3个视野,计算肾间质纤维组织相对面积。肾间质纤维组织相对面积=肾小球内Masson阳性染色面积/肾小球总面积×100%[7]。
1.5 免疫组织化学染色检测2组幼鼠肾小球中RhoA和ROCK蛋白表达水平将5 μm石蜡切片60℃烤片1 h,脱蜡至水。枸橼酸盐溶液高压修复10 min,3%H2O2,室温孵育10 min,滴加RhoA一抗(1:100)和ROCK一抗(1:200),4℃过夜,滴加HRP抗兔IgG,孵育20 min,DAB显色。以PBS代替一抗作为阴性对照。每张切片选3个视野,采用Imagepro Plus 6.0软件分析肾小球内棕黄色区域的积分吸光度(IA)值[8],以IA值代表蛋白表达水平。
1.6 免疫印迹法检测2组幼鼠肾小球中RhoA、ROCK和Vimentin蛋白表达水平将2组幼鼠肾组织剪碎离心后取上清。灌胶,加样,电泳,转膜,5%脱脂奶粉室温封闭,加入RhoA(1:2 000)、ROCK(1:2 000)、Vimentin(1:1 000),GAPDH(1:1 000),4℃过夜。二抗室温孵育2 h,ECL显色。采用Fluorchem V2.0系统进行IA值测定,计算目的蛋白表达水平。目的蛋白表达水平=目的蛋白平均IA值/GAPDH平均IA值。
1.7 统计学分析采用SPSS 25.0统计软件进行统计学分析。2组幼鼠体质量,血清TC和TG水平,肾小球系膜和基底膜相对面积,肾间质纤维组织相对面积,肾小球中RhoA、ROCK和Vimentin蛋白表达水平均符合正态分布,以x±s表示,两组间样本均数比较采用独立样本t检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 2组幼鼠体质量将3周龄幼鼠分别喂养4、8和12周后,HFD组幼体型偏胖,体质量增长幅度较快,与正常对照组比较小鼠体质量明显增加(P < 0.01)。见表 1。
| (n=6, x±s) | |||||||||||||||||||||||||||||
| Group | Body weight | ||||||||||||||||||||||||||||
| (week) 0 | 4 | 8 | 12 | ||||||||||||||||||||||||||
| Normal control | 11.58±0.51 | 21.11±0.66 | 27.38±0.62 | 32.45±0.52 | |||||||||||||||||||||||||
| HFD | 11.76±0.36 | 27.76±0.56* | 36.96±0.79* | 47.52±1.24* | |||||||||||||||||||||||||
| * P < 0.01 vs normal control group. | |||||||||||||||||||||||||||||
3周龄幼鼠喂养12周血液生化指标检测结果显示:HFD组幼鼠血清TC水平为(1.84±010)mmol·L-1,正常对照组幼鼠血清TC水平为(0.86±0.09)mmol·L-1;HFD组幼鼠血清TG水平为(1.45±0.07)mmol·L-1,正常对照组幼鼠血清TG水平为(0.87±0.10)mmol·L-1;HFD组幼鼠血清TC和TG水平较正常对照组升高(P < 0.01)。
2.3 2组幼鼠肾组织病理形态表现3周龄幼鼠喂养12周后,与正常对照组比较,HFD组幼鼠肾小球内出现明显脂肪变性,PAS染色呈强阳性,肾小球系膜基质轻度增生; 与正常对照组(28%±3%)比较,HFD组幼鼠肾小球肾小球系膜和基底膜相对面积(55%±1%)明显增大(P < 0.05)。Masson染色显示HFD组幼鼠肾小球内蓝色胶原纤维染色物质比正常对照组增多; 与正常对照组(16.97%±0.85%)比较,HFD组幼鼠肾间质纤维组织相对面积(24.84%±3.32%)明显增大,P < 0.05)。见图 1(插页五)。
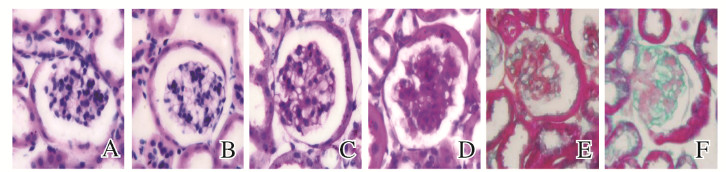
|
| A, B: HE staining; C, D: PAS staining; E, F: Masson staining.A, C, E:Normal control group; B, D, F:HFD group. 图 1 2组幼鼠肾组织病理形态表现(×400) Fig. 1 Pathomorphology of kidney tissue of young mice in two groups(×400) |
|
|
RhoA和ROCK蛋白均表达在系膜细胞的细胞膜和细胞浆中。与正常对照组(0.065±0.007,0.071±0.013)比较,HFD组幼鼠肾小球中RhoA(0.092±0.008)和ROCK蛋白(0.116±0.020)表达水平升高(P < 0.05)。见图 2(插页五)。
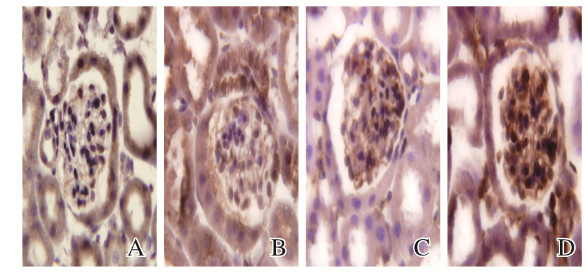
|
| A, B: RhoA; C, D: ROCK; A, C: Normal control group; B, D: HFD group. 图 2 2组幼鼠肾小球中RhoA和ROCK蛋白表达(免疫组织化学,×400) Fig. 2 Expressions of RhoA and ROCK proteins in glomeruli of young mice in two groups(Immunochemistry, ×400) |
|
|
RhoA、ROCK和Vimentin蛋白的相对分子质量分别为22 000、15 800和57 000。HFD组幼鼠肾组织中RhoA、ROCK和Vimentin蛋白表达水平均高于正常对照组(P < 0.05)。见图 3和表 2。
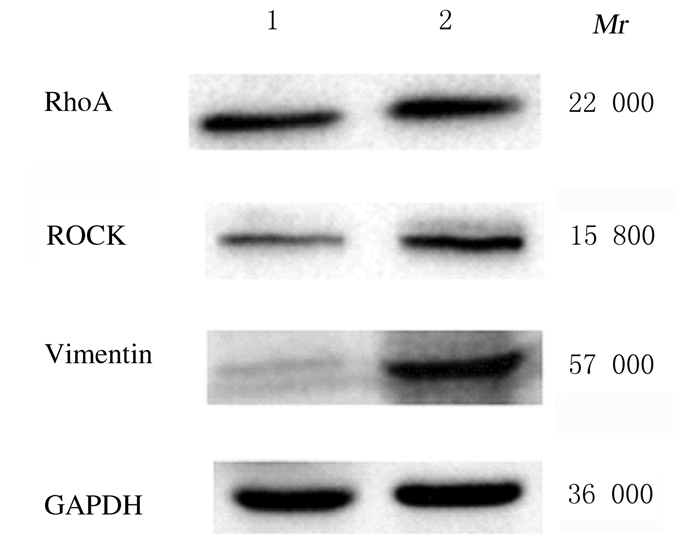
|
| Lane 1: Normal control group; Lane 2: HFD group. 图 3 2组幼鼠肾组织中RhoA、ROCK和Vimentin蛋白表达电泳图 Fig. 3 Electrophoregram of RhoA, ROCK, and Vimentin proteins in kidney tissue of young mice in two groups |
|
|
| (n=6, x±s) | |||||||||||||||||||||||||||||
| Group | RhoA | ROCK | Vimentin | ||||||||||||||||||||||||||
| Normal control | 0.96±0.15 | 0.99±0.28 | 0.29±0.05 | ||||||||||||||||||||||||||
| HFD | 1.55±0.20* | 2.01±0.39* | 1.27±0.21* | ||||||||||||||||||||||||||
| * P < 0.05 vs normal control group. | |||||||||||||||||||||||||||||
研究[9]表明:肥胖和一系列与脂质代谢紊乱有关的疾病均与HFD有密切关系。与肥胖有关的多种慢性肾脏疾病通常会出现肾小球肥大、系膜基质增生、蛋白尿和肾纤维化等症状[10-11]。RhoA能被多种细胞因子和炎症活化从而激活RhoA/ROCK通路,参与这些细胞因子介导的多种炎症损伤与纤维组织增生性疾病的病理生理过程[12]。ROCK属于丝氨酸/苏氨酸蛋白激酶家族成员,ROCK信号参与了许多依赖细胞骨架的生理活动,包括细胞黏附、运动、吞噬以及细胞-细胞和细胞-基质黏附[13]。RhoA /ROCK信号通路对维持肾小球结构和功能的稳定性具有重要意义。研究[14]显示:RhoA/ ROCK通路在高糖下被激活,同时伴有肾小球系膜基质增多以及出现蛋白尿,应用ROCK抑制剂则明显改善肾脏纤维化,降低蛋白尿。因此,本研究旨在探讨RhoA/ROCK通路在HFD所致肾小球损伤中的作用及意义。
RhoA/ROCK信号通路对肾小球系膜细胞起到关键性作用。肾小球系膜细胞是肾小球的固有细胞,具有吞噬、促增殖和收缩等功能,同时还能分泌细胞外基质,产生细胞因子及生长因子[15]。在病理情况下系膜细胞可产生大量的细胞外基质,细胞外基质过度堆积会造成肾小球基底膜增厚、系膜基质扩张,逐渐发展为肾小球硬化和纤维化[16]。KOLAVENNU等[17]发现:高糖刺激可激活体外培养肾小球系膜细胞RhoA/ROCK信号通路,促使肌动蛋白形成,并增加Ⅳ型胶原蛋白的合成。体外实验[21]进一步证实:抑制ROCK可以减少糖尿病db/db鼠肾小球基底膜的胶原积聚,降低蛋白尿。还有学者[18]发现:高糖能上调人肾小球系膜细胞中RhoA的表达,从而激活其下游的ROCK、细胞外基质成分以及致纤维化因子的表达;而ROCK抑制剂可通过抑制RhoA/ROCK通路的活性,减少肾小球的纤维化和炎症反应。PENG等[19]采用ROCK抑制剂法舒地尔治疗STZ糖尿病大鼠,发现通过抑制ROCK的活性,可引起肾小球系膜基质积聚和肾小球纤维连接蛋白表达减少。本课题组前期实验[22]表明:小鼠于出生6周后肾小球发育成熟。因此,本文作者选用肾小球未发育成熟的3周龄C57BL/6J幼鼠作为研究对象,观察幼鼠体质量、血液生化指标和肾组织形态表现是否因为HFD的诱导而产生改变,结果显示:HFD组幼鼠体型比正常对照组小鼠偏胖,HFD组幼鼠血脂和胆固醇水平呈现升高的趋势,表明HFD组幼鼠形成肥胖和代谢指标异常。本研究结果显示:HFD组幼鼠肾小球出现了脂肪变性、系膜基质增生以及胶原纤维染色物质增多;HFD组幼鼠肾小球中Vimentin蛋白表达水平明显高于正常对照组。Vimentin属于细胞骨架蛋白,是间质细胞中的重要中间纤维,也是肾纤维化的标志蛋白之一[20],提示HFD组幼鼠肾脏出现了轻度纤维化的改变。免疫组织化学和蛋白质印迹检测结果显示:与正常对照组比较,HFD组幼鼠肾组织中RhoA和ROCK蛋白表达均明显增强,HFD可能造成肾脏损伤,而RhoA/ROCK通路参与了该过程,且RhoA和ROCK蛋白的高表达可能通过作用于系膜细胞,导致系膜基质增生和纤维积聚,从而造成肾小球损伤。
综上所述,RhoA/ROCK信号通路可能参与了HFD诱导的肾小球损伤,但其确切的发生机制还有待进行深入研究和探索。
| [1] |
AN YN, LI Y, WANG X Y, et al. Cordycepin reduces weight through regulating gut microbiota in high-fat diet-induced obese rats[J]. Lipids Health Dis, 2018, 17(1): 276. DOI:10.1186/s12944-018-0910-6 |
| [2] |
YU O M, BROWN J H. G protein-coupled receptor and RhoA-stimulated transcriptional responses: links to inflammation, differentiation, and cell proliferation[J]. Mol Pharmacol, 2015, 88(1): 171-180. |
| [3] |
MONAGHAN-BENSON E, WITTCHEN E S, DOERSCHUK CM, et al. A Rnd3/p190RhoGAP pathway regulates RhoA activity in idiopathic pulmonary fibrosis fibroblasts[J]. Mol Biol Cell, 2018, 29(18): 2165-2175. DOI:10.1091/mbc.E17-11-0642 |
| [4] |
MANICKAM N, PATEL M, GRIENDLING K K, et al. RhoA/Rho kinase mediates TGF-β1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species[J]. Am J Physiol Renal Physiol, 2014, 307(2): F159-F171. DOI:10.1152/ajprenal.00546.2013 |
| [5] |
FENG S, ZOU L, WANG H J, et al. RhoA/ROCK-2 pathway inhibition and tight junction protein upregulation by catalpol suppresses lipopolysaccaride-induced disruption of blood-brain barrier permeability[J]. Molecules, 2018, 23(9): E2371. DOI:10.3390/molecules23092371 |
| [6] |
TAKIYAMA Y, SERA T, NAKAMURA M, et al. Impacts of diabetes and an SGLT2 inhibitor on the glomerular number and volume in db/db mice, as estimated by synchrotron radiation micro-CT at spring-8[J]. EBioMedicine, 2018, 36: 329-346. DOI:10.1016/j.ebiom.2018.09.048 |
| [7] |
WU W, HU W, HAN W B, et al. Inhibition of Akt/mTOR/p70S6K signaling activity with huangkui capsule alleviates the early glomerular pathological changes in diabetic nephropathy[J]. Front Pharmacol, 2018, 9: 443. DOI:10.3389/fphar.2018.00443 |
| [8] |
BAO J F, SHI Y F, TAO M, et al. Pharmacological inhibition of autophagy by 3-MA attenuates hyperuricemic nephropathy[J]. Clin Sci, 2018, 132(21): 2299-2322. DOI:10.1042/CS20180563 |
| [9] |
KOVESDY C P, FURTH S L, ZOCCALI C. Obesity and kidney disease: hidden consequences of the epidemic[J]. Braz J Med Biol Res, 2017, 50(5): e6075. |
| [10] |
BARTON M, SOROKIN A. Endothelin and the glomerulus in chronic kidney disease[J]. Semin Nephrol, 2015, 35(2): 156-167. |
| [11] |
NIU H L, LI Y, LI H B, et al. Matrix metalloproteinase 12 modulates high-fat-diet induced glomerular fibrogenesis and inflammation in a mouse model of obesity[J]. Sci Rep, 2016, 6: 20171. DOI:10.1038/srep20171 |
| [12] |
JERUSCHKE S, BÜSCHER A K, OH J, et al. Protective effects of the mTOR inhibitor everolimus on cytoskeletal injury in human podocytes are mediated by RhoA signaling[J]. PLoS One, 2013, 8(2): e55980. DOI:10.1371/journal.pone.0055980 |
| [13] |
BABA I, EGI Y, UTSUMI H, et al. Inhibitory effects of fasudil on renal interstitial fibrosis induced by unilateral ureteral obstruction[J]. Mol Med Rep, 2015, 12(6): 8010-8020. |
| [14] |
刘恒.Rho/Rho激酶信号通路和肝细胞生长因子在2型糖尿病大鼠肾病中的作用[D].石家庄: 河北医科大学, 2012. http://d.wanfangdata.com.cn/Thesis/Y2106188
|
| [15] |
XIE X, CHEN Q, TAO J. Astaxanthin promotes Nrf2/ARE signaling to inhibit HG-induced renal fibrosis in GMCs[J]. Mar Drugs, 2018, 16(4): 117. DOI:10.3390/md16040117 |
| [16] |
谌祖江.芪丹地黄汤抑制糖尿病肾病肾脏纤维化的作用和机制研究[D].广州: 南方医科大学, 2018.
|
| [17] |
KOLAVENNU V, ZENG L X, PENG H, et al. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control[J]. Diabetes, 2008, 57(3): 714-723. DOI:10.2337/db07-1241 |
| [18] |
马东蔚, 王秋月, 马小羽. 法舒地尔通过Rho/ROCK信号通路对高糖培养人肾小球系膜细胞炎症反应及纤维化的影响[J]. 中华内科杂志, 2011, 50(7): 580-584. DOI:10.3760/cma.j.issn.0578-1426.2011.07.012 |
| [19] |
PENG F F, WU D C, GAO B, et al. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease[J]. Diabetes, 2008, 57(6): 1683-1692. DOI:10.2337/db07-1149 |
| [20] |
DANIELSSON F, PETERSON M K, CALDEIRA ARAUJO H, et al. Vimentin diversity in health and disease[J]. Cells, 2018, 7(10): E147. DOI:10.3390/cells7100147 |
| [21] |
SUBATHRA M, KORRAPATI M, HOWELL L A, et al. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells[J]. Am J Physiol Renal Physiol, 2015, 309(3): F204-F215. DOI:10.1152/ajprenal.00150.2015 |
| [22] |
沈健, 臧东钰, 李晓明. 高脂饮食诱导的C57BL/6J幼鼠肾足细胞中Synaptodin的表达及意义[J]. 解放军医学院学报, 2015, 36(9): 940-943. DOI:10.3969/j.issn.2095-5227.2015.09.022 |
 2019, Vol. 45
2019, Vol. 45


