扩展功能
文章信息
- 史素楠, 刘浩, 宫亮
- SHI Sunan, LIU Hao, GONG Liang
- 不同周龄小鼠耳蜗组织中Otos的表达及其意义
- Expressions of Otos in cochlear tissue of mice at different ages and their significances
- 吉林大学学报(医学版), 2019, 45(06): 1299-1304
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1299-1304
- 10.13481/j.1671-587x.20190618
-
文章历史
- 收稿日期: 2019-05-31
年龄相关性听力损失(age-related hearing loss, AHL)又称老年性聋,是人类最常见的听力损伤类型,随着人口老龄化的加速,AHL发病率逐年上升。根据最新全球疾病负担(GBD)结果,AHL已成为影响全球老年人健康的主要因素,由其所致的交流障碍、社会孤立状态和劳动能力下降给全球带来了巨大的经济损失和社会负担,因此AHL越来越受到国内外学者关注[1-2]。许多因素会促进AHL的发生发展,总体而言,内耳细胞的变性和缺失是AHL的主要原因。内耳细胞包括感觉毛细胞、螺旋神经元、耳蜗侧壁的血管纹和纤维细胞等非感觉细胞,其中非感觉细胞不仅能给感觉毛细胞提供必需的支持,而且对于维持正常听觉功能有重要意义[3-6]。Otos是新发现的特异性表达于耳蜗螺旋缘、螺旋韧带和前庭上皮下层区域的基因,敲除该基因后内耳感觉毛细胞和血管纹显示正常,但出现纤维细胞变性、内淋巴离子紊乱,甚至出现中度耳聋[7]。众多学者[8-9]推测Otos可能具有保护螺旋韧带纤维细胞和维持耳蜗内环境稳定的作用。但该基因在耳蜗组织中的表达水平是否与年龄具有相关性尚未见报道。本研究通过观察不同周龄C57BL/6Cnc小鼠耳蜗组织中Otos及其编码的蛋白Otospiralin的表达与分布, 分析该基因与AHL的关系及其在正常听觉生理中的作用。
1 材料与方法 1.1 实验动物、主要试剂和仪器40只SPF级健康C57BL/6Cnc小鼠购自北京维通利华实验动物技术有限公司,动物合格证号:SCXK(京)2016-0006。适应性饲养1周后开始实验。鼠Otospiralin单克隆抗体、Alexa Fluor® 594标记山羊抗鼠IgG和HRP标记山羊抗鼠IgG(美国Santa Cruz公司),BCA蛋白定量试剂盒(北京碧云天生物有限公司),动物组织总RNA提取试剂盒(北京天根公司),还原第一链cDNA合成试剂盒(RevertAid First Strand cDNA Synthesis Kit,美国Thermo Scientific公司),Power SYBR® Green PCR Master Mix(美国Thermo Fisher Scientific公司)。听觉诱发电位-耳声发射记录系统(SmartEP&OAE, 美国IHS公司),冰冻切片机(CM3050S,德国Leica公司)和激光共聚焦显微镜(Leica TCS sp5Ⅱ,德国Leica公司),荧光定量PCR(qRT-PCR)仪(Quant Studio3,美国Thermo Fisher Scientific公司)。
1.2 各组小鼠听性脑干反应(auditory brainstem response, ABR)检查所有小鼠按鼠龄分为4周龄组、16周龄组、32周龄组和48周龄组,每组10只,雌雄各半,鼓膜完整,无中耳炎病史,无噪声、耳毒性药物接触史。以1%戊巴比妥钠(90 mg·kg-1)对各组小鼠进行腹腔注射麻醉,麻醉生效后分别将电极正极置于颅顶正中皮下, 负极连接被测耳, 接地电极连接对侧耳。SmartEP&OAE听觉诱发电位-耳声发射记录系统给予短纯音刺激, 刺激声经插入式耳塞给入,选取12000、24000和32000 Hz 3个频率分别进行测试并记录ABR阈值。重复率为39.1 beats·s-1, 带通滤波100~3 000 Hz, 叠加1 024次, 扫描时程16.0 ms[10]。声刺激强度从80 dB SPL开始, 以10 dB逐次递减, 接近听阈时以5 dB递减,最后一次出现ABRⅠ波并与上一波形有延续性的声强数值定为ABR阈值,实验至少重复3次。同一小鼠各频率ABR阈值的平均值为该鼠ABR平均阈值。
1.3 各组小鼠耳蜗冰冻切片的制备各组小鼠行ABR测试后,断头取听泡并分离耳蜗,显微镜下分离镫骨,打开前庭窗和蜗窗,以4%多聚甲醛溶液(pH 7.4)固定过夜,随后将标本置于10% EDTANa2溶液中脱钙3~7 d,以20%和30%蔗糖溶液梯度脱水后OCT包埋,沿蜗轴水平行冰冻切片,片厚约8 μm。切片晾干后-80℃保存。
1.4 免疫荧光法检测各组小鼠耳蜗组织中Otospiralin蛋白表达量和分布从-80℃冰箱取出切片,复温至室温, PBS清洗3次, 10%山羊血清封闭1h,鼠Otospiralin单克隆抗体(1:500)4℃孵育过夜。PBS清洗3次,Alexa Fluor® 594标记山羊抗鼠二抗(1:500)室温避光孵育1h,PBS清洗3次, 抗荧光衰减封片剂封片,激光共聚焦显微镜下观察结果并拍照。阴性对照染色切片用PBS代替一抗, 其他实验步骤不变。应用Image-Proplus6.0软件测量各组阳性产物的吸光度(A)值。A值越大表示目的蛋白表达量越高。
1.5 qRT-PCR法检测各组耳蜗组织中Otos mRNA表达水平各组小鼠断头后立即于冰上取出耳蜗,迅速放入预冷的TRIzol中,分别提取各组小鼠耳蜗总RNA,然后逆转录合成cDNA。PCR反应体系: cDNA 2 μL, 上下游引物各1μL (10 μmol·L-1), Power SYBR® Green PCR Master Mix 12.5 μL,补DEPC水至25 μL。q-RT-PCR反应条件为:95℃、30 s; 95℃、5 s, 60℃、30 s,40个循环。本反应以小鼠耳蜗组织中GAPDH mRNA为内参基因,读取目的基因及内参基因的循环阈值(Ct值),并计算目的基因与内参基因Ct值差值(ΔCt),以2-ΔΔCt值代表目的基因mRNA表达水平。Otos引物序列由大连宝生物有限公司设计。见表 1。
| Gene | Sequence(5′-3′) | Length(bp) | Temperature(θ/℃) | Size(bp) |
| Otos -F Otos -R | CCAGCAGAGAAACAGAGACCTACA GGCTGGGACAAGGAAGGAA | 24 19 | 62.17 62.48 | 95 |
| GAPDH-F GAPDH-R | AAATGGTGAAGGTCGGTGTGAAC CAACAATCTCCACTTTGCCACTG | 23 23 | 64.40 64.00 | 90 |
各组小鼠断头后迅速分离耳蜗,5个耳蜗为一份样本,加入RIPA裂解液及蛋白酶抑制剂(100:1)提取耳蜗组织蛋白,BCA法测定蛋白浓度。每组20μL蛋白样本SDS-PAGE后转膜,封闭,加入鼠Otospiralin单克隆抗体(1:100)并4℃过夜。TBST洗膜,辣根过氧化物酶标记的山羊抗鼠二抗(1:3 000)室温孵育1h,TBST洗膜后ECL法发光显影。采用ImageJ软件分析条带灰度,以β-actin作为内参蛋白,计算Otospiralin蛋白表达水平。Otospiralin蛋白表达水平=Otospiralin蛋白灰度值/β-actin蛋白灰度值。
1.7 统计学分析采用SPSS 17.0统计软件进行统计学分析。各组小鼠ABR平均阈值、耳蜗组织中Otospiralin蛋白表达量、耳蜗组织中Otospiralin mRNA和Otospiralin蛋白表达水平均以x±s表示,数据符合正态分布和方差齐性,多组间样本均数比较采用单因素方差分析,组间两两比较采用LSD法。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠ABR平均阈值在12000、24000和32000 Hz频率的刺激下,4、16、32和48周龄组小鼠ABR平均阈值分别为(16.17±2.61)、(34.33±5.62)、(56.83±3.55)和(68.17±5.35)dB SPL。16、32和48周龄组小鼠ABR平均阈值较4周龄组均明显升高(P<0.01),32和48周龄组小鼠ABR平均阈值较16周龄组均明显升高(P<0.01),48周龄组小鼠ABR平均阈值较32周龄组明显升高(P<0.01)。
2.2 各组小鼠耳蜗组织中Otospiralin蛋白表达量和分布Otospiralin蛋白在耳蜗组织中主要表达于螺旋韧带,且在螺旋韧带5种纤维细胞中均有表达。与4周龄组比较,16、32和48周龄组小鼠耳蜗组织中Otospiralin蛋白表达量均明显降低(P<0.01);与16周龄组比较,32和48周龄组小鼠耳蜗组织中Otospiralin蛋白表达量均明显降低(P<0.01);与32周龄组比较,48周龄组小鼠耳蜗组织中Otospiralin蛋白表达量明显降低(P<0.01)。见图 1(插页五)和表 2。
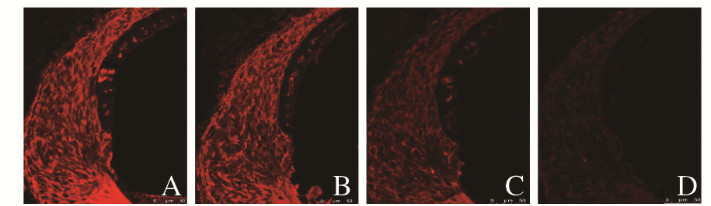
|
| A:4-week-old group; B:16-week-old group; C:32-week-old group; D:48-week-old group. 图 1 各组小鼠耳蜗组织中Otospiralin蛋白表达情况(免疫荧光,×630) Fig. 1 Expressions of Otospiralin in cochlea tissue of mice in various groups(Immunofluorescence, ×630) |
|
|
| (n=3, x±s) | |||||||||||||||||||||||||||||
| Group | Expression amount of Otospiralin protein | Expression level of Otos mRNA | Expression level of Otospiralin protein | ||||||||||||||||||||||||||
| 4-week-old | 0.064±0.001 | 1.007±0.003 | 0.596±0.007 | ||||||||||||||||||||||||||
| 16-week-old | 0.054±0.002* | 0.815±0.020* | 0.411±0.009* | ||||||||||||||||||||||||||
| 32-week-old | 0.031±0.001*△ | 0.511±0.025*△ | 0.287±0.006*△ | ||||||||||||||||||||||||||
| 48-week-old | 0.013±0.001*△# | 0.313±0.010*△# | 0.190±0.014*△# | ||||||||||||||||||||||||||
| * P < 0.01 vs 4-week-old group; △ P < 0.01 vs 16-week-old group; # P < 0.01 vs 32-week-old group. | |||||||||||||||||||||||||||||
与4周龄组比较,16、32和48周龄组小鼠耳蜗组织中Otos mRNA表达水平均明显降低(P<0.01);与16周龄组比较,32和48周龄组小鼠耳蜗组织中OtosmRNA表达水平均明显降低(P<0.01);与32周龄组比较,48周龄组小鼠耳蜗组织中Otos mRNA表达水平明显降低(P<0.01)。见表 2。
2.4 各组小鼠耳蜗组织中Otospiralin蛋白表达水平各组小鼠耳蜗组织中Otospiralin条带结果与免疫荧光结果一致,随着鼠龄的增长,Otospiralin蛋白表达水平逐渐降低。与4周龄组比较,16、32和48周龄组小鼠耳蜗组织中Otospiralin蛋白表达水平降低(P < 0.01);与16周龄组比较,32和48周龄组小鼠耳蜗组织中Otospiralin蛋白表达水平降低(P < 0.01);与32周龄组比较,48周龄组小鼠蜗组织中Otospiralin蛋白表达水平降低(P < 0.01)。见图 2和表 2。
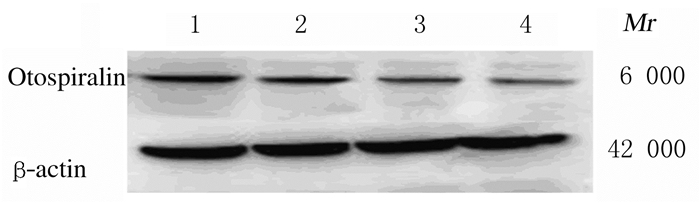
|
| Lane 1:4-week-old group; Lane 2:16-week-old group; Lane 3:32-week-old group; Lane 4:48-week-old group; Lane 1-4:4-, 8-, 16-, and 32-week-old groups. 图 2 各组小鼠耳蜗组织中Otospiralin蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of Otospiralin protein in cochlea tissue of mice in various groups |
|
|
Otos是近年来在大鼠cDNA耳蜗基因库中新发现的一种基因,现仅在内耳组织和大脑组织中检测到微量的Otos mRNA及其蛋白,其他组织中均未见表达。荧光原位杂交证实Otos位于2号染色体q37.3位点,跨越1 630个核苷酸,包含4个外显子和567个核苷酸的cDNA编码。Otos编码的蛋白质为Otospiralin,相对分子质量约为6 400,主要由耳蜗螺旋韧带和螺旋缘的纤维细胞以及前庭感觉上皮下基质分泌。有学者[11-16]对Otospiralin序列分析发现Otospiralin仅一小部分(12个残基)与C型逆转录病毒gap p30核衣壳蛋白的N端存在同源序列,这12个残基是鱼类和哺乳动物非常保守的16个氨基酸片段的一部分,由此学者[11-16]推测该蛋白在维持耳蜗正常结构和功能中发挥至关重要的作用。
Otospiralin主要参与耳蜗螺旋韧带构成,既往螺旋韧带被认为仅仅起结构支持的机械作用,近年研究[9, 17-19]显示:螺旋韧带参与构成血迷路屏障,维持内耳钾离子循环和耳蜗内电位(endocochlear potential, EP),在维持正常听觉生理中起至关重要作用,因此螺旋韧带逐渐成为耳科学研究的热点。螺旋韧带上表达多种蛋白,如Na-K-ATPase、Na-K-2Cl联合转运体、Cx26和Cx30等,这些蛋白表达异常可以引起耳蜗内淋巴离子紊乱,EP下降,最终出现听力损失[3, 17, 20-21]。目前Na-K-ATPase和Na-K-2Cl联合转运体已被证明参与AHL的发生。然而Otos的表达水平是否与年龄具有相关性尚未见报道,基于以上信息,本文作者假设:Otos在耳蜗组织中表达水平随着鼠龄增长而逐渐减少,并且该变化参与AHL的发生发展。
本研究检测各周龄C57BL/6Cnc小鼠的ABR平均阈值,观察Otos mRNA及其蛋白在不同周龄小鼠耳蜗组织中的表达水平结果表明:Otospiralin在正常小鼠耳蜗组织中主要表达于螺旋韧带,且在螺旋韧带5种纤维细胞中均有表达。随着C57BL/6Cnc小鼠鼠龄的增长,其ABR平均阈值逐渐升高,Otos mRNA和Otospiralin蛋白表达水平均逐渐减少。由此可推断Otos的表达水平不仅与年龄具有相关性,而且可能在AHL的发生发展中起一定作用。但目前Otos在内耳组织中维持正常听觉功能的具体机制尚不明确,其表达水平减少通过何种信号通路影响听力有待后续研究。
除参与AHL的发生外,Otos还被认为具有保护内耳纤维细胞和恢复受损听觉功能的作用。研究[9, 22]显示:一些应激性因素如噪声、耳毒性药物损伤耳蜗后引起内耳细胞凋亡和听力减退。与此同时,螺旋韧带纤维细胞发生有丝分裂,以Ⅱ型和Ⅳ型纤维细胞增殖最为显著,甚至有些动物模型会出现听力损失后再恢复的现象。目前纤维细胞再生的机制尚未可知[23-24]。有实验[7, 25]表明Otos基因敲除鼠的耳蜗螺旋韧带Ⅱ型和Ⅳ型纤维细胞发生变性,而毛细胞和血管纹正常,这表明Otos对纤维细胞本身具有一定保护作用。ZHUO等[9]证实:上调Otos的表达水平对顺铂损伤的螺旋韧带纤维细胞具有保护作用,Otos是否在内耳损伤后参与纤维细胞增殖和修复有待进一步研究。同时由于Otos在内耳中表达的特异性, 探索Otos在内耳中的作用机制也将为耳聋的基因治疗提供新思路。
综上所述,随着C57BL/6Cnc小鼠鼠龄的增加,其听觉功能逐渐减退,Otos mRNA和Otospiralin蛋白表达水平也逐渐减少, Otos的这种改变可能参与AHL的发生发展。本研究为治疗和预防AHL提供了新的研究方向。
| [1] |
UCHIDA Y, SUGIURA S, NISHITA Y, et al. Age-related hearing loss and cognitive decline-The potential mechanisms linking the two[J]. Auris Nasus Larynx, 2019, 46(1): 1-9. DOI:10.1016/j.anl.2018.08.010 |
| [2] |
李胜利, 武坤毅, 任晓勇. 年龄相关听力损失DBA/2J小鼠耳蜗毛细胞马达蛋白-prestin表达下调与耳聋的关系[J]. 中华耳科学杂志, 2018, 16(4): 562-569. DOI:10.3969/j.issn.1672-2922.2018.04.025 |
| [3] |
LANG H N, JYOTHI V, SMYTHE N M, et al. Chronic reduction of endocochlear potential reduces auditory nerve activity: further confirmation of an animal model of metabolic presbyacusis[J]. J Assoc Res Otolaryngol, 2010, 11(3): 419-434. DOI:10.1007/s10162-010-0214-7 |
| [4] |
KEITHLEY ELIZABETH M.Pathology and mechanisms of cochlear aging.[J].Neurosci Res, 2019: 1-11.https://doi.org/10.1002/jnr.24439.
|
| [5] |
KITAO K, MIZUTARI K, NAKAGAWA S, et al. Recovery of endocochlear potential after severe damage to lateral wall fibrocytes following acute cochlear energy failure[J]. Neuroreport, 2016, 27(15): 1159-1166. DOI:10.1097/WNR.0000000000000673 |
| [6] |
FETONI A R, PICCIOTTI P M, PALUDETTI G, et al. Pathogenesis of presbycusis in animal models: a review[J]. Exp Gerontol, 2011, 46(6): 413-425. DOI:10.1016/j.exger.2010.12.003 |
| [7] |
DELPRAT B, RUEL J, GUITTON M J, et al. Deafness and cochlear fibrocyte alterations in mice deficient for the inner ear protein otospiralin[J]. Mol Cell Biol, 2005, 25(2): 847-853. DOI:10.1128/MCB.25.2.847-853.2005 |
| [8] |
STEVENS S M, XING Y Z, HENSLEY C T, et al. Heptanol application to the mouse round window:a model for studying cochlear lateral wall regeneration[J]. Otolaryngol Head Neck Surg, 2014, 150(4): 659-665. DOI:10.1177/0194599813518876 |
| [9] |
ZHUO X L, WANG Y, ZHUO W L, et al. Adenoviral-mediated up-regulation of Otos, a novel specific cochlear gene, decreases cisplatin-induced apoptosis of cultured spiral ligament fibrocytes via MAPK/mitochondrial pathway[J]. Toxicology, 2008, 248(1): 33-38. |
| [10] |
马婷婷, 党嫣, 贾培丽, 等. 葛根素对顺铂致毒小鼠耳蜗钙蛋白酶2表达的影响[J]. 听力学及言语疾病杂志, 2019, 27(1): 58-62. DOI:10.3969/j.issn.1006-7299.2019.01.014 |
| [11] |
TORKTAZ I, BEHJATI M, ROSTAMI A. Phylogenetic analysis of otospiralin protein[J]. Adv Biomed Res, 2016, 5: 41. DOI:10.4103/2277-9175.178787 |
| [12] |
CARAVELLI A, PIANESE L, SAULINO C, et al. Down-regulation of otospiralin mRNA in response to acoustic stress in guinea pig[J]. Hear Res, 2004, 198(1/2): 36-40. |
| [13] |
KAMIYA K, FUJINAMI Y, HOYA N, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes[J]. Am J Pathol, 2007, 171(1): 214-226. |
| [14] |
DECOURT B, HILLMAN D, BOULEAU Y, et al. Is otospiralin inner ear specific? Evidence for its expression in mouse brain[J]. Int J Dev Neurosci, 2009, 27(1): 87-96. |
| [15] |
DELPRAT B, BOULANGER A, WANG J, et al. Downregulation of otospiralin, a novel inner ear protein, causes hair cell degeneration and deafness[J]. J Neurosci, 2002, 22(5): 1718-1725. DOI:10.1523/JNEUROSCI.22-05-01718.2002 |
| [16] |
LAVIGNE-REBILLARD M, DELPRAT B, SURGET M O, et al. Gene structure, chromosomal localization, and mutation screening of the human gene for the inner ear protein otospiralin[J]. Neurogenetics, 2003, 4(3): 137-140. |
| [17] |
DING B, WALTON J P, ZHU X X, et al. Age-related changes in Na, K-ATPase expression, subunit isoform selection and assembly in the stria vascularis lateral wall of mouse cochlea[J]. Hear Res, 2018, 367: 59-73. DOI:10.1016/j.heares.2018.07.006 |
| [18] |
KURATA N, SCHACHERN P A, PAPARELLA M M, et al. Histopathologic evaluation of vascular findings in the cochlea in patients with presbycusis[J]. JAMA Otolaryngol Head Neck Surg, 2016, 142(2): 173-178. DOI:10.1001/jamaoto.2015.3163 |
| [19] |
PATUZZI R. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential[J]. Hear Res, 2011, 277(1/2): 4-19. |
| [20] |
KELLY J J, ABITBOL J M, HULME S, et al. The connexin 30 A88V mutant reduces cochlear gap junction expression and confers long-term protection against hearing loss[J]. J Cell Sci, 2019, 132(2): jcs224097. DOI:10.1242/jcs.224097 |
| [21] |
DEFOURNY J, THELEN N, THIRY M. Actin-independent trafficking of cochlear connexin 26 to non-lipid raft gap junction plaques[J]. Hear Res, 2019, 374: 69-75. DOI:10.1016/j.heares.2019.01.020 |
| [22] |
NOBLE K V, REYZER M L, BARTH J L, et al. Use of proteomic imaging coupled with transcriptomic analysis to identify biomolecules responsive to cochlear injury[J]. Front Mol Neurosci, 2018, 11: 243. DOI:10.3389/fnmol.2018.00243 |
| [23] |
HAO X P, XING Y Z, MOORE M W, et al. Sox10 expressing cells in the lateral wall of the aged mouse and human cochlea[J]. PLoS One, 2014, 9(6): e97389. DOI:10.1371/journal.pone.0097389 |
| [24] |
STEVENS S M, BROWN L N, EZELL P C, et al. The mouse round-window approach for ototoxic agent delivery: a rapid and reliable technique for inducing cochlear cell degeneration[J]. J Vis Exp, 2015(105). DOI:10.3791/53131 |
| [25] |
GARCÍA BERROCAL J R, MÉNDEZ-BENEGASSI I, MARTÍ C, et al. Intervention of spiral ligament fibrocytes in the metabolic regulation of the inner ear[J]. Acta Otorrinolaringol Esp, 2008, 59(10): 494-499. DOI:10.1016/S0001-6519(08)75519-5 |
 2019, Vol. 45
2019, Vol. 45


