扩展功能
文章信息
- 李金花, 金颖, 李俊峰, 李莉
- LI Jinhua, JIN Ying, LI Junfeng, LI Li
- 芝麻素对AD模型小鼠学习和记忆能力的影响及其机制
- Effects of sesamin on abilities of learning and memory in AD model mice and their mechanisms
- 吉林大学学报(医学版), 2019, 45(06): 1275-1280
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1275-1280
- 10.13481/j.1671-587x.20190614
-
文章历史
- 收稿日期: 2019-09-14
2. 延边大学医学院解剖学教研室, 吉林 延吉 133002
2. Department of Anatomy, School of Medical Sciences, Yanbian University, Yanji 133002, China
阿尔茨海默病(Alzheimer’ s disease,AD)是老年痴呆最常见的原因之一。AD的特征为淀粉样β蛋白(amyloid β-protein,Aβ)沉积于神经炎性斑块、细胞内神经原纤维缠结中tau蛋白蓄积和神经元丢失[1-3]。Aβ可通过多种途径促使神经细胞产生氧化应激状态,产生大量活性氧,从而诱导神经细胞凋亡,最终导致神经细胞减少并出现认知功能障碍等。目前,可用于治疗AD的药物疗效有限且不能被治愈,所以AD防治药物的研发是一个急需解决的问题。芝麻素是从芝麻籽油中提取的一种木脂素,常被用作一种强效的抗氧化剂,研究[4-5]表明:芝麻油能够抑制脂多糖诱导的神经胶质细胞炎症细胞因子的释放,通过其抗氧化特性在脑缺血疾病中发挥神经保护作用,近年来研究[6-7]显示芝麻素可抑制β-分泌酶活性。但芝麻素的氧化应激调控机制对AD影响的相关研究报道较少。本研究采用淀粉样β蛋白片段25-35(amyloid β-protein fragment 25-35, Aβ25-35)诱导建立AD小鼠模型,采用不同浓度芝麻素进行干预,观察其对AD小鼠学习和记忆能力的影响,并对小鼠氧化应激指标和凋亡相关蛋白水平进行检测,阐明芝麻素对AD小鼠学习和记忆功能改善作用的可能机制。
1 材料与方法 1.1 动物、主要试剂和仪器雄性C57BL/6小鼠40只,体质量(20±2) g,由延边大学医学院实验动物中心提供。芝麻素(上海宝曼生物科技有限公司),吡拉西坦(上海广锐生物科技有限公司),Aβ25-35(美国Sigma公司),DCFH-DA探针(南京建成生物工程研究所),超氧化物歧化酶(superoxide dismutase,SOD)和丙二醛(malondialdehyde,MDA)试剂盒(上海信裕生物科技有限公司),B淋巴细胞瘤2(B-cell lymphoma-2,Bcl-2)、Bcl-2相关X蛋白(Bcl-2 associated X protein, Bax)、含半胱氨酸的天冬氨酸蛋白水解酶3(cysteine aspartate specific proteinase-3, Caspase-3)和β-actin一抗(美国Cell Signaling Technology公司),辣根过氧化物酶(HRP二抗,美国Earthox公司)。Morris水迷宫(上海玉研科学仪器有限公司),单细胞悬液仪(上海净信实业发展有限公司),Cyto FLEX流式细胞仪(贝克曼库尔特商贸中国有限公司),AI600凝胶成像仪(美国GE公司)。
1.2 小鼠AD模型的建立和给药小鼠适应性饲养1周后随机分为对照组、模型组、吡拉西坦组、低剂量芝麻素组和高剂量芝麻素组,每组8只。各组小鼠采用戊巴比妥钠麻醉后定位,自前囱后2 mm、矢状缝右1.5 mm为右侧脑室;模型组、吡拉西坦组、低和高剂量芝麻素组小鼠采用微量注射器沿定位位置刺入深度约为2 mm,以2 μL·min-1的速度注入3 μL Aβ25-35(浓度为2 mmol·L-1),对照组小鼠注入3 μL生理盐水。7 d后,吡拉西坦组小鼠用吡拉西坦150 mg·kg-1灌胃,低和高剂量芝麻素组小鼠分别用50和100 mg·kg-1芝麻素灌胃,对照组和模型组小鼠给予等量生理盐水灌胃。各组小鼠每天给药1次,共2周。
1.3 Morris水迷宫实验检测各组小鼠逃避潜伏期给药2周后小鼠进行Morris水迷宫实验。①定位航行实验:将小鼠放置于某一象限的平台,从对角象限的起始点将小鼠背对水面置于水中,采用录像设备记录小鼠找到平台的时间和移动路线。当小鼠游到平台上或在2 min内未找到平台,实验结束。小鼠找到平台的时间即逃避潜伏期,2 min内未游到者按2 min计。小鼠在平台休息30 s后进行下一次实验,连续测试4 d。②空间探索实验:实验第5天将平台撤去,在目标象限的对角象限起始点将小鼠背对水面放于水中,利用录像设备记录小鼠2 min内的游泳时间和路线,记录目标象限游程及目标象限停留时间。
1.4 各组小鼠脑组织中活性氧(active oxygen,ROS)水平测定水迷宫实验结束后处死小鼠取脑,弃去小脑和嗅球,剩余脑组织用单细胞悬液仪制备单细胞悬液,加入DCFH-DA,37℃孵育1 h后1 000 g离心5 min,将细胞重悬于PBS中,采用Cyto FLEX流式细胞仪检测其荧光强度,以平均荧光强度计算各组小鼠脑组织中ROS水平,小鼠脑组织中ROS水平=实验组平均荧光强度/对照组平均荧光强度×100%。
1.5 各组小鼠脑组织中SOD活性和MDA水平检测取小鼠脑组织进行匀浆,按照试剂盒说明书采用ELISA法检测小鼠脑组织中SOD活性和MDA水平。
1.6 Western blotting检测各组小鼠脑组织进行匀浆后提取蛋白,采用BAC法进行蛋白定量,上样总蛋白40 μg,90 V电压进行电泳分离,300 mA电流转膜1 h,利用5%脱脂奶粉封闭1 h,TBST清洗后加入Bcl-2、Bax、Caspase-3和β-actin一抗,4℃孵育过夜。第二天用HRP标记二抗孵育2 h,ECL发光后在AI 600凝胶成像仪中进行曝光,并记录实验结果。Image J图像分析软件测定目的蛋白条带的灰度值,计算目的蛋白表达水平。目的蛋白表达水平=目的蛋白条带灰度值/β-actin灰度值。
1.7 统计学分析采用SPSS17.0统计软件进行统计学分析。各组小鼠水迷宫实验中的逃避潜伏期、目标象限游程和目标象限时间,脑组织中ROS水平、SOD活性和MDA水平,脑组织中Bcl-2、Bax和Caspase-3蛋白表达水平均以x±s表示,以上各指标多组间比较采用单因素方差分析,组间两两比较采用LSD法。以P<0.05为差异有统计学意义。
2 结果 2.1 Morris水迷宫实验中各组小鼠小鼠逃避潜伏期在小鼠定位航行实验中,AD小鼠逃避潜伏期随着训练时间的延长逐渐缩短。第1~4天,与对照组比较,AD小鼠逃避潜伏期明显延长(P<0.05);与模型组比较,吡拉西坦组和各剂量芝麻素组小鼠逃避潜伏期明显缩短(P<0.05)。见表 1。
| (n=8, x±s, t/s) | |||||||||||||||||||||||||||||
| Group | Escape latency | ||||||||||||||||||||||||||||
| (t/d) 1 | 2 | 3 | 4 | ||||||||||||||||||||||||||
| Control | 89.36±5.61 | 85.25±9.14 | 76.36±6.21 | 51.65±5.41 | |||||||||||||||||||||||||
| Model | 118.84±1.52* | 106.41±9.62* | 97.48±9.47* | 82.49±8.25* | |||||||||||||||||||||||||
| Piracetam | 94.65±4.37△ | 91.58±8.15△ | 84.74±8.36△ | 67.61±6.58△ | |||||||||||||||||||||||||
| Sesamin | |||||||||||||||||||||||||||||
| Low dose | 115.26±6.15△ | 101.48±8.59△ | 92.42±6.69△ | 84.25±8.63△ | |||||||||||||||||||||||||
| High dose | 102.74±5.17△ | 98.13±7.69△ | 85.97±8.54△ | 79.62±7.25△ | |||||||||||||||||||||||||
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组小鼠目标象限游程和目标象限停留时间明显缩短(P<0.05);与模型组比较,吡拉西坦组和各剂量芝麻素组小鼠目标象限游程和目标象限停留时间增加(P<0.05)。见表 2。
| (n=8, x±s) | |||||||||||||||||||||||||||||
| Group | Swimming distance(l/cm) | Resident time(t/s) | |||||||||||||||||||||||||||
| Control | 586.47±13.65 | 26.69±2.69 | |||||||||||||||||||||||||||
| Model | 508.65±11.52* | 21.36±2.14* | |||||||||||||||||||||||||||
| Piracetam | 536.74±14.37△ | 25.82±2.31△ | |||||||||||||||||||||||||||
| Sesamin | |||||||||||||||||||||||||||||
| Low dose | 512.57±18.15△ | 22.49±2.36△ | |||||||||||||||||||||||||||
| High dose | 522.49±11.17△ | 23.58±2.78△ | |||||||||||||||||||||||||||
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
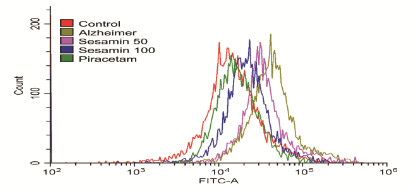
流式细胞术检测结果显示:AD小鼠脑组织中ROS水平曲线明显右移,量化后ROS水平明显高于对照组(P<0.05);吡拉西坦组和各剂量芝麻素组小鼠脑组织中ROS曲线左移,ROS水平较模型组明显降低(P<0.05)。见图 1(插页四)和表 3。
|
| 图 1 流式细胞术检测各组小鼠脑组织中ROS水平 Fig. 1 Levels of ROS in brain tissue of mice in various groups detected by flow cytometry |
|
|
| (n=8, x±s,η/%) | |||||||||||||||||||||||||||||
| Group | Level of ROS | ||||||||||||||||||||||||||||
| Control | 100.00±9.12 | ||||||||||||||||||||||||||||
| Model | 262.45±16.24* | ||||||||||||||||||||||||||||
| Piracetam | 106.37±9.76△ | ||||||||||||||||||||||||||||
| Sesamin | |||||||||||||||||||||||||||||
| Low dose | 201.12±15.48△ | ||||||||||||||||||||||||||||
| High dose | 121.89±11.89△ | ||||||||||||||||||||||||||||
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组小鼠脑组织中SOD活性明显降低(P < 0.05),MDA水平明显升高(P < 0.05)。与模型组比较,吡拉西坦组和各剂量芝麻素组小鼠脑组织中SOD活性明显升高(P < 0.05),MDA水平明显降低(P < 0.05)。见表 4。
| (n=8, x±s) | |||||||||||||||||||||||||||||
| Group | SOD[λB/(U·mg-1)] | MDA[cB/(nmol·L-1)] | |||||||||||||||||||||||||||
| Control | 0.60±0.06 | 0.47±0.16 | |||||||||||||||||||||||||||
| Model | 0.38±0.05* | 0.84±0.12* | |||||||||||||||||||||||||||
| Sesamin | |||||||||||||||||||||||||||||
| Low dose | 0.44±0.02△ | 0.72±0.13△ | |||||||||||||||||||||||||||
| High dose | 0.52±0.04△ | 0.61±0.14△ | |||||||||||||||||||||||||||
| Piracetam | 0.57±0.06△ | 0.54±0.12△ | |||||||||||||||||||||||||||
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
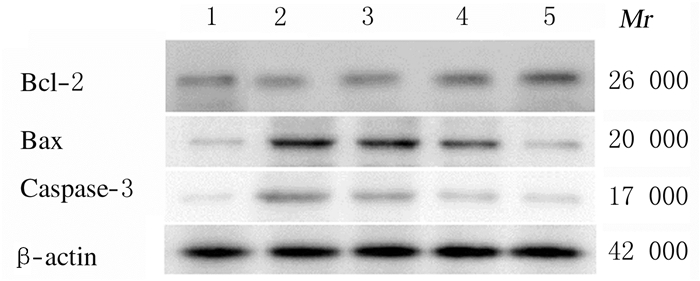
与对照组比较,模型组小鼠脑组织中Bcl-2蛋白表达水平明显降低(P < 0.05),Bax、Caspase-3蛋白表达水平和Bcl-2/ Bax比值明显升高(P < 0.05)。与模型组比较,吡拉西坦组和各剂量芝麻素组小鼠脑组织中Bcl-2蛋白表达水平和Bcl-2/ Bax比值升高(P < 0.05),Bax和Caspase-3蛋白表达水平降低(P < 0.05)。见图 2和表 5。
|
| Lane 1:Control group; Lane 2: Model group; Lane 3 :Low dose of sesamin group; Lane 4:High dose of sesamin group; Lane 5:Piracetam group. 图 2 Western blotting法检测各组小鼠脑组织中Bcl-2、Bax和Caspase-3蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of Bcl-2, Bax and Caspase-3 proteins in brain tissue of mice in various groups detected by Western blotting method |
|
|
| (n=8, x±s) | |||||||||||||||||||||||||||||
| Group | Bcl-2 | Bax | Caspase-3 | Bcl-2/ Bax | |||||||||||||||||||||||||
| Control | 0.56±0.05 | 0.28±0.01 | 0.17±0.01 | 2.12±0.23 | |||||||||||||||||||||||||
| Model | 0.28±0.02* | 0.72±0.04* | 0.46±0.03* | 0.39±0.02* | |||||||||||||||||||||||||
| Piracetam | 0.52±0.05△ | 0.29±0.01△ | 0.18±0.01△ | 1.79±0.16△ | |||||||||||||||||||||||||
| Sesamin | |||||||||||||||||||||||||||||
| Low dose | 0.36±0.03△ | 0.58±0.03△ | 0.32±0.03△ | 0.62±0.08△ | |||||||||||||||||||||||||
| High dose | 0.45±0.04△ | 0.42±0.02△ | 0.21±0.02△ | 1.07±0.10△ | |||||||||||||||||||||||||
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
氧化应激在脑功能障碍发生发展中起重要作用[8-9]。氧化应激条件下,机体ROS大量产生并积聚,致使蛋白质和DNA损伤,诱发炎症反应和细胞凋亡[10]。脑内ROS的产生可引起神经元损伤并导致患者认知功能降低和行为抑制,参与AD、帕金森病、衰老和许多其他神经疾病的发生[11-12]。在AD患者发病早期相关病理变化出现之前神经元中就出现了氧化性损伤,氧化损伤可能是促进AD发生的重要机制[13]。近年来研究[14]表明芝麻素通过抑制氧化应激减少神经毒性化学物质诱导的神经元损伤,并且在SOD1缺失的果蝇中,芝麻素可抑制多巴胺神经元数量随衰老的下降。此外有研究[15]显示摄入芝麻素可以改善人类的认知功能。本研究结果显示:芝麻素可明显缩短AD小鼠逃避潜伏期,增加目标象限游程和目标象限时间。同时芝麻素能够提高小鼠脑组织中SOD活性,降低ROS和MDA水平,提示芝麻素能够提高AD小鼠的学习记忆能力,其作用可能与抗氧化应激能力有关。
ROS是氧化应激的重要指标,研究[16]表明ROS介导的包括海马区在内的神经元凋亡增加可能是AD发生的一个重要机制。神经元发生凋亡时多种凋亡途径被启动,导致Caspase-3激活。Caspase-3作用于Caspase前体、细胞骨架蛋白和Bcl-2蛋白家族等,使细胞进入凋亡执行阶段。Bcl-2家族分为凋亡诱导剂(Bax的头部)和凋亡抑制剂(Bcl-2的头部)两大类[17],是凋亡过程中的关键因素。当Bax表达升高形成同源二聚体时诱导细胞凋亡,Bax与Bcl-2形成异源二聚体时则抑制细胞凋亡。Bcl-2/Bax比值是启动细胞凋亡的“分子开关”。相关实验[18-19]也证实了Bcl-2表达水平降低和Bax表达水平升高是高血糖介导的诱导神经细胞凋亡毒性的关键因素。本研究结果显示:AD小鼠脑组织中Caspase-3和Bax蛋白表达水平升高并伴随Bcl-2蛋白表达水平降低,Bcl-2/Bax比值明显降低。芝麻素能明显降低AD小鼠脑组织中Bax和Caspase-3蛋白表达水平,提高Bcl-2蛋白表达水平,上调Bcl-2/Bax比值进而影响细胞凋亡。
综上所述,芝麻素可能通过抑制氧化应激诱导的细胞凋亡而改善AD模型小鼠学习和记忆能力。
| [1] |
NORTON S, MATTHEWS F E, BARNES D E, et al. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data[J]. Lancet Neurol, 2014, 13(8): 788-794. DOI:10.1016/S1474-4422(14)70136-X |
| [2] |
SCHELTENS P, BLENNOW K, BRETELER M M, et al. Alzheimer's disease[J]. Lancet, 2016, 388: 505-517. DOI:10.1016/S0140-6736(15)01124-1 |
| [3] |
WILLIAMS P, SORRIBAS A, HOWES M J. Natural products as a source of Alzheimer's drug leads[J]. Nat Prod Rep, 2011, 28(1): 48-77. DOI:10.1039/C0NP00027B |
| [4] |
JENG K C, HOU R C, WANG J C, et al. Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-actived protein kinase and nuclear factor kappa B[J]. Immunol Lett, 2005, 97(1): 101-106. DOI:10.1016/j.imlet.2004.10.004 |
| [5] |
MIYAWAKI T, AONO H, TOYODA-ONO Y, et al. Antihypertensive effects of sesamin in humans[J]. J Nutr Sci Vitaminol, 2009, 55(1): 87-91. |
| [6] |
MATSUMURA S, MURATA K, YOSHIOKA Y, et al. Search for β-Secretase inhibitors from natural spices[J]. Nat Prod Commun, 2016, 11(4): 507-510. |
| [7] |
MURATA K, MATSUMURA S, YOSHIOKA Y, et al. Screening of β-secretase and acetylcholinesterase inhibitors from plant resources[J]. J Nat Med, 2015, 69(1): 123-129. DOI:10.1007/s11418-014-0859-3 |
| [8] |
SAKUL A, CUMAOGALU A, AYDIN E, et al. Age- and diabetes-induced regulation of oxidative protein modification in rat brain and peripheral tissues: Consequences of treatment with antioxidant pyridoindole[J]. Exp Gerontol, 2013, 48(5): 476-484. DOI:10.1016/j.exger.2013.02.028 |
| [9] |
KISHIDO T, UNNO K, YOSHIDA H, et al. Decline in glutathione peroxidase activity is a reason for brain senescence: Consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain[J]. Biogerontology, 2007, 8(4): 423-430. DOI:10.1007/s10522-007-9085-7 |
| [10] |
赵永军. 运动、活性氧与阿尔茨海默病[J]. 体育科学研究, 2010, 14(4): 90-94. DOI:10.3969/j.issn.1007-7413.2010.04.021 |
| [11] |
WALTON J C, SELVAKUMAR B, WEIL Z M, et al. Neuronal nitric oxide synthase and NADPH oxidase interact to affect cognitive, affective, and social behaviors in mice[J]. Behav Brain Res, 2013, 320-327. |
| [12] |
SEO J S, PARK J Y, CHOI J, et al. NADPH oxidase mediates depressive behavior induced by chronic stress in mice[J]. J Neurosci, 2012, 32(28): 9690-9699. DOI:10.1523/JNEUROSCI.0794-12.2012 |
| [13] |
OTEIZA P I. A mechanism for the stimulatory effect of aluminum on iron-induced lipid peroxidation[J]. Arch Biochem Biophys, 1994, 308(2): 374-379. DOI:10.1006/abbi.1994.1053 |
| [14] |
付剑亮, 邵福源. 阿尔茨海默病发病机制研究进展[J]. 世界临床药物, 2010, 31(7): 390-394. |
| [15] |
LE T D, NAKAHARA Y, UEDA M, et al. Sesamin suppresses aging phenotypes in adult muscular and nervous systems and intestines in a Drosophila senescence-accelerated model[J]. Eur Rev Med Pharmacol Sci, 2019, 23(4): 1826-1839. |
| [16] |
ITO N, SAITO H, SEKI S, et al. Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mild cognitive impairment: A randomized, double-blind, placebo-controlled trial[J]. J Alzheimers Dis, 2018, 62(4): 1767-1775. DOI:10.3233/JAD-170969 |
| [17] |
KROEMER G. The proto-oncogene Bcl-2 and its role in regulating apoptosis[J]. Nat Med, 1997, 3(6): 614-620. DOI:10.1038/nm0697-614 |
| [18] |
JAFARI ANARKOOLI I, SANKIAN M, AHMADPOUR S, et al. Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats[J]. Exp Diabetes Res, 2008, 2008: 638467. |
| [19] |
JAFARI ANARKOOLI I, SANKIAN M, VAHEDI F, et al. Evaluation of insulin and ascorbic acid effects on expression of Bcl-2 family proteins and caspase-3 activity in hippocampus of STZ-induced diabetic rats[J]. Cell Mol Neurobiol, 2009, 29(1): 133-140. DOI:10.1007/s10571-008-9305-y |
 2019, Vol. 45
2019, Vol. 45


