扩展功能
文章信息
- 房惠, 杨红宇, 马绪哲, 陈立松, 盖晓东, 历春
- FANG Hui, YANG Hongyu, MA Xuzhe, CHEN Lisong, GAI Xiaodong, LI Chun
- 叉头蛋白3对肺腺癌细胞增殖和化疗药物顺铂敏感性的影响
- Effects of FOXP3 on cell proliferation and chemosensitivity to cisplatin in lung adenocarcinoma cells
- 吉林大学学报(医学版), 2019, 45(06): 1261-1266
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1261-1266
- 10.13481/j.1671-587x.20190612
-
文章历史
- 收稿日期: 2018-12-23
2. 吉化集团公司总医院病理科, 吉林 吉林 132001;
3. 北华大学医学院病理教研室, 吉林 吉林 132013
2. Department of Pathology, General Hospital of Jihua Group, Jilin 132021, China;
3. Department of Pathology, College of Medical Sciences, Beihua University, Jilin 132013, China
肺腺癌占非小细胞肺癌的40%~50%,其发病率逐年升高,且进展快,预后差。目前化疗仍是治疗肺腺癌最有效的手段之一,肿瘤细胞对化疗药物敏感性降低是影响化疗疗效和患者生存率的主要原因之一。因此,研究肿瘤细胞对化疗药物敏感性降低的相关机制是肿瘤治疗学研究的热点。叉头蛋白3(forkhead box protein 3,FOXP3)是CD4+CD25+调节性T细胞(regulatory T cells,Tregs)特异的标志物,可通过发挥免疫抑制作用促进肿瘤的恶性进展,影响患者的预后[1-4]。研究[5-10]显示:FOXP3在肿瘤细胞中也有表达,且在不同的肿瘤细胞中其功能各不相同,但均与肿瘤的发生发展和预后有密切关联。本文作者前期研究[11-12]显示:FOXP3高表达可促进肺癌细胞的侵袭和转移,降低肺癌细胞对阿霉素和丝裂霉素C的敏感性,表明肺癌细胞FOXP3可能参与化疗药物的抵抗。顺铂是临床治疗晚期肺腺癌的一线化疗药物,因易形成耐药而严重影响其疗效,FOXP3是否参与顺铂耐药目前尚未见报道。本研究首先检测FOXP3在肺腺癌组织中的表达水平及其与Ki-67的相关性,通过小干扰RNA(siRNA)方法沉默肺腺癌A549细胞中FOXP3基因的表达,观察FOXP3对A549细胞增殖和顺铂敏感性的影响,进一步检测顺铂作用A549细胞后FOXP3表达的变化,旨在明确FOXP3在顺铂耐药中的作用,为提高肺腺癌的化疗疗效提供有效的分子靶点。
1 材料与方法 1.1 一般资料收集2009年3月—2012年12月吉化集团公司总医院病理科原发性肺腺癌术后石蜡标本50例和正常肺组织10例。其中男性26例,女性24例,年龄40~77岁,平均年龄(60.0±7.1)岁。所有患者术前均未接受放化疗,术后均经病理学检测明确诊断。
1.2 细胞培养人肺腺癌细胞株A549由北华大学分子医学实验室保存,细胞用含有10%新生小牛血清的DMEM培养液,置于5% CO2的37℃孵箱中培养。
1.3 主要试剂和仪器DMEM高糖培养液(美国HyClone公司),新生小牛血清(杭州四季青公司),FOXP3 siRNA和Control-siRNA (美国Santa Cruz Biotechnology公司),PrimeScriptTM RT试剂盒和SYBR Premix Ex TaqTM Ⅱ试剂(日本TaKaRa公司),LipofectamineTM 2000试剂盒(美国Invitrogen公司),CCK-8试剂盒(碧云天生物技术有限公司),小鼠抗人FOXP3单克隆抗体(美国Abcam公司),Ki-67即用型抗体和PV-6000通用型免疫组织化学试剂盒(北京中杉生物公司)。Western blotting电泳设备(美国Bio-Rad Laboratories公司),全自动酶标仪(美国Bio-Tek公司),实时荧光定量PCR检测系统(美国Thermo Fisher Scientific Inc公司)。
1.4 免疫组织化学染色和结果判定将石蜡标本重新制备为厚4 μm切片,参照试剂盒说明书进行免疫组织化学染色,以PBS替代一抗作阴性对照。双盲法判定染色结果,以细胞浆或细胞核显示棕黄色颗粒为阳性细胞。FOXP3结果判定依照文献[11],Ki-67结果判定依照文献[13]。
1.5 siRNA转染和分组取对数生长期A549细胞(每孔2.3×105个)接种至6孔板中,转染前将细胞培养基换为不含血清和抗生素的新鲜培养基,按照LipofectamineTM2000试剂盒说明书进行转染。将转染FOXP3-siRNA、Control-siRNA和仅加脂质体的各组细胞分别命名为FOXP3-siRNA组、Control-siRNA组和Mock组。
1.6 RT-qPCR法检测各组A549细胞中FOXP3 mRNA表达水平采用TRIzol法提取各组细胞中总RNA,按照逆转录试剂盒说明书进行逆转录,将得到的cDNA作为模板进行Real-time PCR反应,反应总体积为20 μL。FOXP3引物上游序列为5′-CACAACATGCGACCCCCTTTCACC-3′,下游序列为5′-AGG TTGTGGCGGATGGCGTTCTTC-3′;GAPDH引物上游序列为5′-ATGGGGAAGGTGAAGGTCG-3′,下游序列为5′-GGGTCATTGATGGCAACAATATC-3′。反应条件:95 ℃、15 min,95 ℃、10 s,60 ℃、10 s,共40个循环。以GAPDH为内参,通过2-△△CT方法计算各样本中FOXP3 mRNA表达水平,实验重复3次。
1.7 Western blotting法检测各组A549细胞中FOXP3蛋白表达水平胰蛋白酶消化收集各组细胞,以预冷的PBS洗涤细胞2次。每组细胞加入150 μL RIPA裂解液,4℃孵育30 min,充分裂解细胞。4℃、12 000 r·min-1离心15 min后收集上清液。采用BCA法测定细胞总蛋白浓度,蛋白上样量为50 μg,12% SDS-PAGE电泳1 h,湿转法将胶内蛋白质印迹到PVDF膜上,采用含5%脱脂奶粉的TBST室温封闭膜1 h。弃去封闭液,加入FOXP3抗体(1:500)4℃孵育过夜。次日TBST洗涤3次,加入HRP标记的二抗(1:3 000)室温孵育1 h。TBST洗涤3次,ECL发光并拍照,以GAPDH为内参对各组蛋白进行灰度值分析,每组蛋白重复3次。目的蛋白表达水平=每个样本条带灰度值/GAPDH灰度值。
1.8 CCK-8法检测各组A549细胞增殖活性和生长抑制率将各组细胞以1×103个/孔的密度接种至96孔板中,每孔接种200 μL细胞悬液,每组设3个复孔。细胞增殖实验:共铺3块板,各组细胞分别在培养24、48和72 h后加入CCK-8溶液10 μL/孔,37℃孵育2 h后,测定490 nm处的吸光度(A)值,以A值代表细胞增殖活性,取各组平均A值绘制生长曲线。细胞毒性实验:各组细胞分别加入不同浓度(0、0.63、1.25、2.50和5.00 mg·L-1)顺铂,即为0、0.63、1.25、2.50和5.00mg·L-1顺铂组,培养48 h后加入CCK-8溶液并测定各孔A值,计算细胞生长抑制率。细胞生长抑制率= (对照组A值-给药组A值)/对照组A值×100%。
1.9 统计学分析采用SPSS 17.0统计软件进行统计学分析。肺腺癌组织和正常肺组织中FOXP3的表达阳性率比较采用χ2检验,FOXP3与Ki-67表达水平相关性分析采用Pearson相关分析;各组A549细胞中FOXP3 mRNA和蛋白表达水平、生长曲线相应A值和细胞生长抑制率均以x±s表示,数据符合正态分布且方差齐,多组间样本均数比较采用单因素方差分析,组间比较采用t检验。以P<0.05为差异有统计学意义。
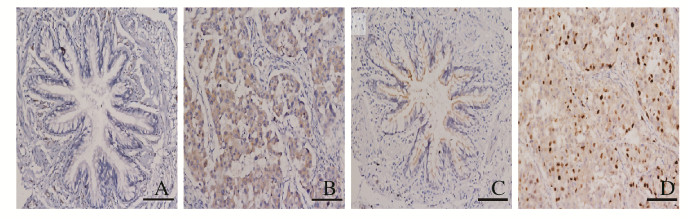
2 结果 2.1 肺腺癌和正常肺组织中FOXP3的表达及其与Ki-67表达的相关性免疫组织化学染色结果显示:FOXP3在肺腺癌组织中呈高表达,在正常肺组织中低表达,阳性表达率分别为62.0%和13.3%,组间比较差异有统计学意义(P < 0.05);31例FOXP3阳性表达标本中Ki-67阳性表达率为74.2%,Pearson相关分析结果表明在肺腺癌组织中FOXP3和Ki-67的表达呈正相关关系(r= 0.370, P < 0.01)。见表 1和图 1(插页三)。
| Ki-67 expression | FOXP3 expression | Total | r | P | |
| + | - | ||||
| + - | 23 8 | 7 12 | 30 20 | 0.370 | 0.008 |
| Total | 31 | 19 | 50 | ||
|
| A, B: FOXP3; C, D: Ki-67;A, C: Normal lung tissue; B, D: Lung adenocacinoma tissue. 图 1 肺腺癌和正常肺组织中FOXP3和Ki-67表达(免疫组织化学,Bar = 100 μm) Fig. 1 Expressions of FOXP3 and Ki-67 in lung adenocarcinoma and normal lung tissues (Immunohistochemistry, Bar = 100 μm) (seen on page 1263in paragraph) A: Example of test result; B: Example of negative reference. |
|
|
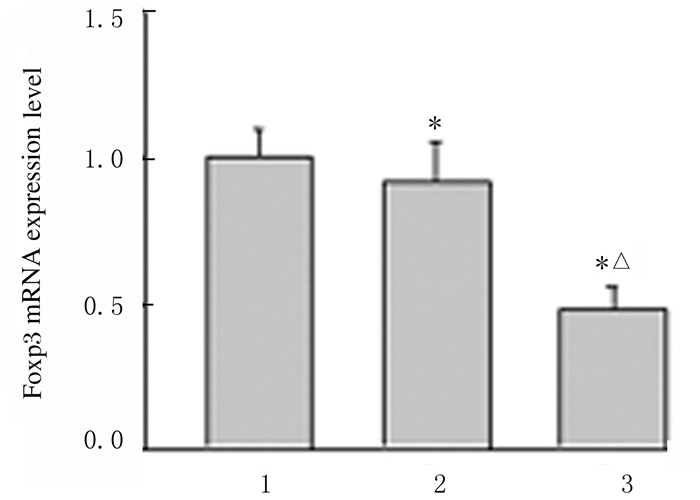
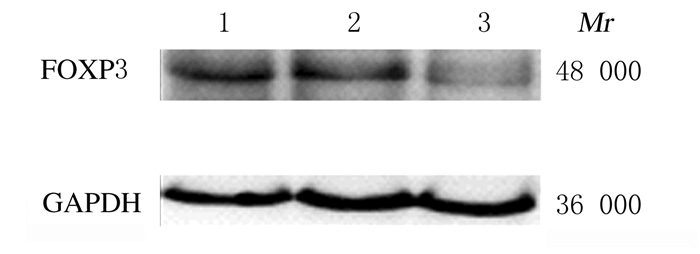
RT-qPCR结果显示:与Mock组和Control-siRNA组比较,FOXP3-siRNA组A549细胞中FOXP3 mRNA表达水平明显降低(P < 0.05),见图 2。Western blotting法检测结果显示:FOXP3-siRNA组A549细胞中FOXP3蛋白相对表达水平为0.58±0.16,明显低于Mock组(1.61±0.22)和Control-siRNA组(1.52±0.30),组间比较差异有统计学意义(P < 0. 05)。见图 3。
|
| Lane 1: Mock group; Lane 2: Control-siRNA group; Lane 3: FOXP3-siRNA group.*P < 0.05 compared with Mock group; △P < 0.05 compared with Control-siRNA group. 图 2 RT-qPCR法检测RNA干扰后各组A549细胞中FOXP3 mRNA表达水平 Fig. 2 Expression levels of FOXP3 mRNA in A549 cells in various groups after RNA interference detected by RT-qPCR method |
|
|
|
| Lane 1: Mock group; Lane 2: Control-siRNA group; Lane 3: FOXP3-siRNA group. 图 3 Western blotting法检测RNA干扰后各组A549细胞中FOXP3蛋白表达电泳图 Fig. 3 Electrophoregram of expressionsof FOXP3 protein in A549 cells in various groups after RNA interference detected by Western blotting method |
|
|
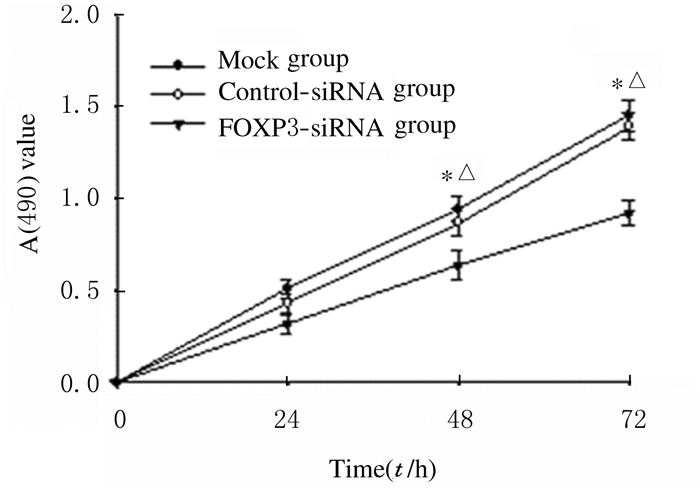
CCK-8法检测结果显示:FOXP3沉默后,A549细胞增殖活性明显降低。在48和72 h时间点,FOXP3-siRNA组细胞增殖活性均低于Mock组和Control-siRNA组(P < 0.05)。见图 4。
|
| *P < 0.05 compared with Mock group; △P < 0.05 compared with Control-siRNA group. 图 4 各组A549细胞增殖活性 Fig. 4 Proliferation activities of A549 cells in various groups |
|
|
CCK-8检测结果显示:顺铂在0.63~5.00 mg·L-1范围内呈浓度依赖性抑制各组A549细胞的增殖。在顺铂浓度为1.25、2.50和5.00 mg·L-1时,与Mock组和Control-siRNA组比较,FOXP3-siRNA组A549细胞生长抑制率明显升高,组间比较差异有统计学意义(P < 0.05)。见表 2。
| (n=3, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | Inhibitory rate of proliferation | ||||||||||||||||||||||||||||
| (mg·L-1)0.63 | 1.25 | 2.50 | 5.00 | ||||||||||||||||||||||||||
| Mock | 27.7±4.14 | 36.5±4.81 | 51.5±5.12 | 76.5±5.49 | |||||||||||||||||||||||||
| Control-siRNA | 29.5±3.68 | 40.0±4.92 | 55.9±4.88 | 78.5±5.30 | |||||||||||||||||||||||||
| FOXP3-siRNA | 34.3±4.19 | 49.5±4.52*△ | 67.6±5.69*△ | 88.0±6.04*△ | |||||||||||||||||||||||||
| * P < 0.05 compared with Mock group; △ P < 0.05 compared with Control-siRNA group. | |||||||||||||||||||||||||||||
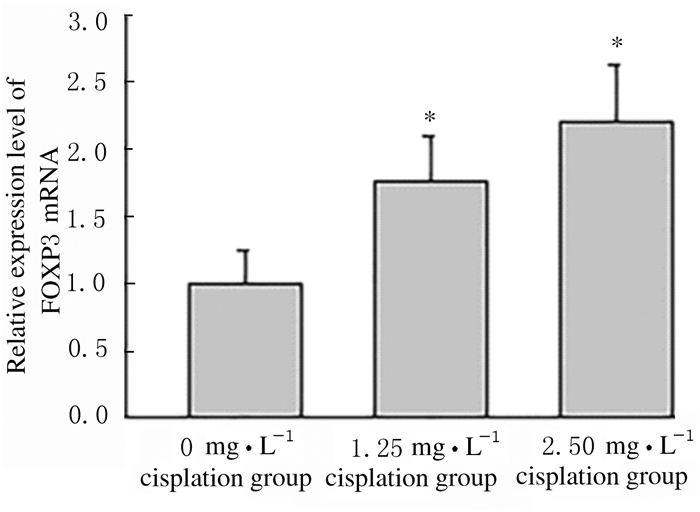
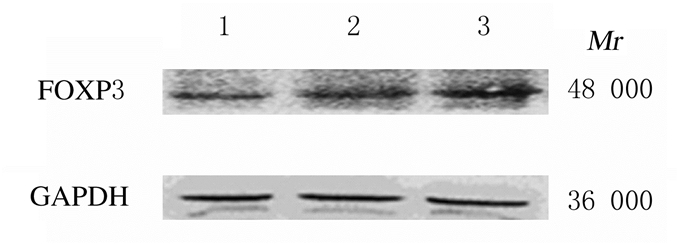
RT-qPCR结果显示:顺铂作用A549细胞48 h后,细胞中FOXP3 mRNA表达水平随顺铂浓度的增加而升高,与0 mg·L-1顺铂组比较差异有统计学意义(P < 0.05),见图 5。Western blotting法检测结果显示:1.25和2.50 mg·L-1顺铂组细胞中FOXP3蛋白表达水平分别为0.75±0.07和0.95±0.12,明显高于0 mg·L-1顺铂组(0.41±0.06),组间比较差异有统计学意义(P < 0.05)。见图 6。
|
| *P < 0.05 compared with 0 mg·L-1 cisplatin group. 图 5 RT-qPCR法检测各组A549细胞中FOXP3 mRNA表达水平 Fig. 5 Expressionlevels of FOXP3 mRNA in A549 cells in various groups detected by RT-qPCR method |
|
|
|
| Lane 1-3:0, 1.25, and 2.50 mg·L-1 cisplatin groups. 图 6 Western blotting法检测各组A549细胞中FOXP3蛋白表达电泳图 Fig. 6 Electrophoregram of expressions of FOXP3 protein in A549 cells in various groups detected by Western blotting method |
|
|
肿瘤细胞对化疗药物敏感性降低是影响化疗疗效和患者预后的重要因素。研究[1-7]表明:FOXP3在Tregs和肿瘤细胞中的表达在肿瘤的发生发展和侵袭转移过程中均发挥了重要作用。此外,胃癌晚期患者在接受新辅助化疗后预后良好者,其体内Tregs数量较化疗前明显减少,表明Tregs与化疗效果有着密切关联[14-15]。本课题组前期研究[12]显示:化疗药物阿霉素和丝裂霉素C作用于FOXP3过表达的小鼠Lewis肺腺癌细胞后,细胞的抑制率随着药物浓度的增加而明显降低,提示FOXP3可能参与化疗药物的抵抗作用。
顺铂是临床肺癌常用的化疗药物,通过影响肺癌细胞DNA合成来抑制肿瘤细胞的分裂与增殖,进而发挥其抗肿瘤作用[16-17],但肺癌细胞对顺铂的耐药性严重影响其疗效。Ki-67是一种与细胞增殖有关的核抗原,是临床中应用最广泛的检测肿瘤细胞增殖活性指标之一,与肺癌患者的预后密切相关[18-20]。本研究首先检测了FOXP3在肺腺癌组织中的表达水平及其与Ki-67的相关性,结果显示FOXP3在肺腺癌组织中呈高表达,其高表达与Ki-67表达呈正相关关系,提示FOXP3可能参与肺腺癌细胞的恶性增殖过程。YANG等[21]发现:FOXP3过表达可明显促进肺癌细胞的增殖,其可能是通过调控原癌基因c-Myc蛋白和细胞周期蛋白Cyclin D1实现。本研究采用siRNA方法沉默肺腺癌A549细胞株中FOXP3的表达,发现FOXP3沉默后肺腺癌细胞的增殖速度明显减慢,进一步证实FOXP3在肺腺癌细胞的恶性增殖过程中发挥重要作用。
为探讨FOXP3在顺铂耐药中的作用,本研究选用不同浓度顺铂作用各组细胞,结果发现各组细胞的生长抑制率均呈浓度依赖性降低。其中,FOXP3-siRNA组细胞生长抑制率较其他2组明显升高,表明FOXP3表达下调可增加顺铂对肺腺癌细胞的杀伤作用,增加肺腺癌细胞对顺铂的敏感性。本研究检测顺铂作用前后FOXP3在A549细胞中表达变化的结果显示:FOXP3表达水平随顺铂药物浓度的增加而升高,表明在顺铂作用后肺腺癌细胞中FOXP3的表达水平升高,但其具体机制还有待深入研究。
FOXP3沉默可明显抑制肺腺癌细胞的增殖,增强肺腺癌细胞对顺铂的敏感性[22-23]。因此,FOXP3可能成为逆转顺铂耐药的新靶点,本研究为临床晚期肺腺癌治疗提供了新的思路。
| [1] |
WHITESIDE TL. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity[J]. Expert Opin Ther Targets, 2018, 22(4): 353-363. DOI:10.1080/14728222.2018.1451514 |
| [2] |
QUE Y, XIAO W, GUAN YX, et al. PD-L1 expression is associated with FOXP3+ regulatory T-cell infiltration of soft tissue sarcoma and poor patient prognosis[J]. J Cancer, 2017, 8(11): 2018-2025. DOI:10.7150/jca.18683 |
| [3] |
KITANO Y, OKABE H, YAMASHITA YI, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma[J]. Br J Cancer, 2018, 118(2): 171-180. DOI:10.1038/bjc.2017.401 |
| [4] |
PENG JZ, YU ZG, XUE L, et al. The effect of foxp3-overexpressing Treg cells on non-small cell lung cancer cells[J]. Mol Med Rep, 2018, 17(4): 5860-5868. |
| [5] |
SUN XW, FENG ZJ, WANG YX, et al. Expression of foxp3 and its prognostic significance in colorectal cancer[J]. Int J Immunopathol Pharmacol, 2017, 30(2): 201-206. DOI:10.1177/0394632017710415 |
| [6] |
BODUC M, ROESSLER M, MANDIC R, et al. Foxp3 expression in lymph node metastases in patients with head and neck cancer[J]. Acta Otolaryngol, 2017, 137(11): 1215-1219. DOI:10.1080/00016489.2017.1353705 |
| [7] |
SONG JJ, ZHAO SJ, FANG J, et al. Foxp3 overexpression in tumor cells predicts poor survival in oral squamous cell carcinoma[J]. BMC Cancer, 2016, 16: 530. DOI:10.1186/s12885-016-2419-6 |
| [8] |
DUAN XH, JIANG B, YANG JH, et al. FOXP3 inhibits MYC expression via regulating miR-198 and influences cell viability, proliferation and cell apoptosis in HepG2[J]. Cancer Med, 2018, 7(12): 6182-6192. DOI:10.1002/cam4.1780 |
| [9] |
LI XJ, GAO Y, LI JJ, et al. FOXP3 inhibits angiogenesis by downregulating VEGF in breast cancer[J]. Cell Death Dis, 2018, 9(7): 744. DOI:10.1038/s41419-018-0790-8 |
| [10] |
LI J P, LIAO X H, XIANG Y, et al. MKL1/miR34a/FOXP3 axis regulates cell proliferation in gastric cancer[J]. J Cell Biochem, 2019, 120(5): 7814-7824. DOI:10.1002/jcb.28056 |
| [11] |
FU HY, LI C, YANG W, et al. FOXP3 and TLR4 protein expression are correlated in non-small cell lung cancer: implications for tumor progression and escape[J]. Acta Histochem, 2013, 115(2): 151-157. DOI:10.1016/j.acthis.2012.06.002 |
| [12] |
LI C, YANG W, GAI XD, et al. Foxp3 overexpression decreases sensitivity to chemotherapy in mouse Lewis lung cancer cells[J]. Mol Med Rep, 2012, 6(5): 977-982. DOI:10.3892/mmr.2012.1066 |
| [13] |
EL-GENDI S, ABU-SHEASHA G. Ki-67 and cell cycle regulators p53, p63 and cyclind1 as prognostic markers for recurrence/progression of bladder urothelial carcinoma[J]. Pathol Oncol Res, 2018, 24(2): 309-322. DOI:10.1007/s12253-017-0250-2 |
| [14] |
HE Q, LI G L, JI X, et al. Impact of the immune cell population in peripheral blood on response and survival in patients receiving neoadjuvant chemotherapy for advanced gastric cancer[J]. Tumour Biol, 2017, 39(5): 1010428317697571. |
| [15] |
LI K, CHEN F C, XIE H J. Decreased FOXP3+ and GARP+ Tregs to neoadjuvant chemotherapy associated with favorable prognosis in advanced gastric cancer[J]. Onco Targets Ther, 2016, 9: 3525-3533. |
| [16] |
ROCHA C R R, SILVA M M, QUINET A, et al. DNA repair pathways and cisplatin resistance: an intimate relationship[J]. Clinics (Sao Paulo), 2018, 73(suppl 1): e478s. |
| [17] |
JAIN A, JAHAGIRDAR D, NILENDU P, et al. Molecular approaches to potentiate cisplatin responsiveness in carcinoma therapeutics[J]. Expert Rev Anticancer Ther, 2017, 17(9): 815-825. DOI:10.1080/14737140.2017.1356231 |
| [18] |
SHEN G H, MA H, PANG F W, et al. Correlations of 18F-FDG and 18F-FLT uptake on PET with Ki-67 expression in patients with lung cancer: a meta-analysis[J]. Acta Radiol, 2018, 59(2): 188-195. DOI:10.1177/0284185117706609 |
| [19] |
HE L Y, ZHANG H, WANG Z K, et al. Diagnostic and prognostic significance of E-cadherin and Ki-67 expression in non-small cell lung cancer patients[J]. Eur Rev Med Pharmacol Sci, 2016, 20(18): 3812-3817. |
| [20] |
WEI D M, CHEN W J, MENG R M, et al. Augmented expression of Ki-67 is correlated with clinicopathological characteristics and prognosis for lung cancer patients: an up-dated systematic review and meta-analysis with 108 studies and 14732 patients[J]. Respir Res, 2018, 19(1): 150. DOI:10.1186/s12931-018-0843-7 |
| [21] |
YANG S C, LIU Y, LI M Y, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer[J]. Mol Cancer, 2017, 16(1): 124. DOI:10.1186/s12943-017-0700-1 |
| [22] |
张志峰, 席维岳. 外周血肿瘤标志物早期诊断肺癌临床价值研究[J]. 中国实用内科杂志, 2018, 38(5): 477-479. |
| [23] |
魏雨晴, 吕镗烽, 刘红兵, 等. 肺癌分子诊断及其研究进展[J]. 中国实用内科杂志, 2019, 39(5): 407-411. |
 2019, Vol. 45
2019, Vol. 45


