扩展功能
文章信息
- 王叶, 王红, 朴丽贞, 杨山, 张书剑, 金正勇
- WANG Ye, WANG Hong, PIAO Lizhen, YANG Shan, ZHANG Shujian, JIN Zhengyong
- 高氧诱导下新生大鼠脑损伤的发生机制和前列腺素E1的干预作用
- Pathogenesis of brain injury induced by hyperoxia in newborn rats and intervention of prostaglandin E1
- 吉林大学学报(医学版), 2019, 45(06): 1206-1211
- Journal of Jilin University (Medicine Edition), 2019, 45(06): 1206-1211
- 10.13481/j.1671-587x.20190603
-
文章历史
- 收稿日期: 2019-04-28
2. 吉林大学中日联谊医院儿科, 吉林 长春 130033
2. Department of Pediatrics, China-Japan Union Hospital, Jilin University, Changchun 130033, China
近年来,随着新生儿重症医疗技术的迅速发展,尤其是一项重要的医疗措施——氧疗的应用,早产儿和新生儿的成活率逐渐升高。因新生儿各组织器官发育不完善,长时间高浓度氧暴露易造成多脏器损伤,如肺损伤[1-3]、早产儿视网膜病变[4]和脑损伤,其中肺和脑是最易累及的器官[5]。目前国内外对高氧导致的肺损伤和眼部损伤非常重视,而对高氧导致的脑损伤研究较少,其发病机制目前尚不明确,有学者[1-3]认为其与细胞凋亡和炎症反应等因素有关。前列腺素E1(prostaglandin E1,PGE-1)具有稳定生物膜、扩张脑血管、抑制中性粒细胞和血小板聚集的作用,进而减少脑血管内皮的损伤,减轻脑水肿,目前关于PGE-1的研究主要集中于高氧肺损伤方面,在高氧脑损伤方面国内外报道较少,PGE-1在高氧脑损伤方面的作用尚不清楚。本研究从内质网应激(endoplasmic reticulum stress, ESR)方面来阐述高氧脑损伤的发病机制,并探讨PGE-1的干预作用,从而为临床治疗提供相关的理论依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器90只新生Wistar大鼠由延边大学动物科提供,动物许可证号:SYXK(吉)(2007-0011),体质量8~10 g,雌雄不限。PGE-1(北京泰德制药股份有限公司),氨基末端蛋白激酶(c-Jun N-terminal protein kinase, JNK)抗体和磷酸化JNK(phosphorylated JNK, p-JNK)抗体(美国Santa Cruz Biotechnology公司)。凝胶成像分析系统(美国Bio-Ra公司)。
1.2 动物分组和模型制备90只新生Wistar大鼠出生后1 d内随机分为对照组、高氧组和高氧+ PGE-1组,每组各30只,雌雄不限。参照文献[6]制备新生大鼠高氧诱导脑损伤模型,将高氧组及高氧+ PGE-1组大鼠放置于自制高氧舱内,舱内为常规压力,吸入医用氧气浓度为100%,氧流量为2 L·min-1,然后使用测氧仪密切监测氧箱内氧浓度,使其氧浓度保持在(85±2)%。动物室内和舱内温度保持在24℃~25℃,湿度维持在60%~65%。将生石灰粉末放置在舱内,作为干燥剂吸收多余的CO2和水蒸气。对照组大鼠置于常温、常压(FIO2=21%)下同一室内正常饲养。每天打开高氧舱5~10 min,打扫舱内卫生,并记录大鼠一般情况和体质量,更换饲料和垫料。高氧+ PGE-1组大鼠自造模第1天起给予PGE-1(2 μg·kg-1·d-1)腹腔注射,连续7 d。对照组和高氧组大鼠同一时间每日腹腔内注射等剂量生理盐水。
1.3 标本采集在造模第1、3和7天,每组随机抽取10只大鼠,将其用乙醚蒸气进行麻醉,将大鼠固定在动物台上,在活体的情况下将头部剪下,立即剥开颅骨,暴露并将脑组织完整取出来。取1/2左脑脑组织用以检测脑组织含水量;右脑脑组织需经4%甲醛溶液心肺灌注后取出,其中1/2右脑脑实质经过脱水、固定等处理,然后进行石蜡包埋和切片,最后进行TUNEL染色和HE染色。剩余的左、右脑组织则在-80℃冰箱内冷冻保存,采用Western blotting法检测脑组织中p-JNK和JNK表达量。
1.4 各组大鼠脑组织含水量测定对制备好的大鼠脑组织进行清洁、称质量,记为脑组织湿质量。置于80℃烤箱内,蒸干水分,每天记录质量,连续2 d称质量保持不变,则记为脑组织的干质量,最后放入37℃烤箱内保存起来。脑组织含水量=(湿质量-干质量)/湿质量。
1.5 各组大鼠脑组织病理形态表现和细胞凋亡指数检测选用甲醛灌注脱水固定的大鼠脑组织,进行石蜡包埋、切片,厚度为3~5 μm,然后将切片放入60℃的恒温箱中,37℃恒温24 h,然后进行脱蜡、水化、染色和封片等步骤,使用形态学显微镜观察各组大鼠脑皮层细胞形态表现。按照TUNEL染色法操作,预处理包埋切片脑组织用于核染色,每组选取4~5张染色切片,应用显微镜观察,记录视野中凋亡细胞,计算脑组织细胞凋亡指数。细胞凋亡指数=凋亡细胞数/总细胞数×100%。
1.6 Western blotting法检测各组大鼠脑组织中p-JNK和JNK表达量将预处理好的脑组织标本进行还原性电泳、转膜、封闭、滴加一抗、二抗等过程。然后将X线片进行曝光后显影、定影、晾干,得到蛋白灰度值和β-action条带灰度值比值,即对应蛋白表达量。
1.7 统计学分析采用SPSS25.0统计软件进行统计学分析。各组大鼠体质量、脑组织含水量、脑组织凋亡指数和脑组织中p-JNK及JNK表达量均符合正态分布,以x±s表示,组间比较采用t检验。以P<0.05为差异有统计学意义。
2 结果 2.1 各组大鼠的体质量第1、3和7天,与对照组比较,高氧组大鼠体质量降低(t=9.19,t=6.62,t=22.19,P<0.05);同一时间,与高氧组比较,高氧+ PGE-1组大鼠体质量明显升高(t=3.86,t=2.84,t=10.17,P<0.05)。见表 1。
| (n=10, x±s, m/g) | |||||||||||||||||||||||||||||
| Group | Body weight | ||||||||||||||||||||||||||||
| (t/d) 1 | 3 | 7 | |||||||||||||||||||||||||||
| Control | 6.50±0.40 | 10.80±0.40 | 17.90±0.60 | ||||||||||||||||||||||||||
| Hyperoxia | 5.20±0.20* | 8.35±1.10* | 12.84±0.40* | ||||||||||||||||||||||||||
| Hyperoxia+ PGE-1 | 5.64±0.30△ | 9.40±0.40△ | 14.90±0.50△ | ||||||||||||||||||||||||||
| * P<0.05 compared with control group;△ P<0.05 compared with hyperoxia group. | |||||||||||||||||||||||||||||
与对照组比较,第1、3和7天高氧组大鼠脑组织含水量增加(t=6.15, t=38.18, t=67.67,P<0.05);与高氧组比较,相同时间高氧+PGE-1组大鼠脑组织含水量降低(t=5.59, t=32.42, t=28.46,P<0.05)。见表 2。
| (n=10, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | Water content | ||||||||||||||||||||||||||||
| (t/d) 1 | 3 | 7 | |||||||||||||||||||||||||||
| Control | 72.9±0.4 | 73.4±0.4 | 73.6±0.3 | ||||||||||||||||||||||||||
| Hyperoxia | 74.0±0.4* | 78.8±0.2* | 84.3±0.4* | ||||||||||||||||||||||||||
| Hyperoxia+ PGE-1 | 73.0±0.4△ | 75.9±0.2△ | 79.8±0.3△ | ||||||||||||||||||||||||||
| * P<0.05 compared with control group;△ P<0.05 compared with hyperoxia group. | |||||||||||||||||||||||||||||
实验第1、3和7天时对照组大鼠大脑皮层细胞形态规则,排列有序,极少有炎性细胞的浸润和细胞水肿。高氧组大鼠皮层细胞细胞形态不规则,排列紊乱,胞质淡染水肿,胞核偏大,大量核固缩,血管壁扩张充血,核深染的炎性细胞多;第7天时细胞形态改变更明显。与高氧组比较,高氧+PGE-1组大鼠大脑皮层细胞排列整齐,细胞形态相对规则,核质比有所改善,脑实质内炎性细胞明显减少,脑水肿减轻。见图 1(插页一)。
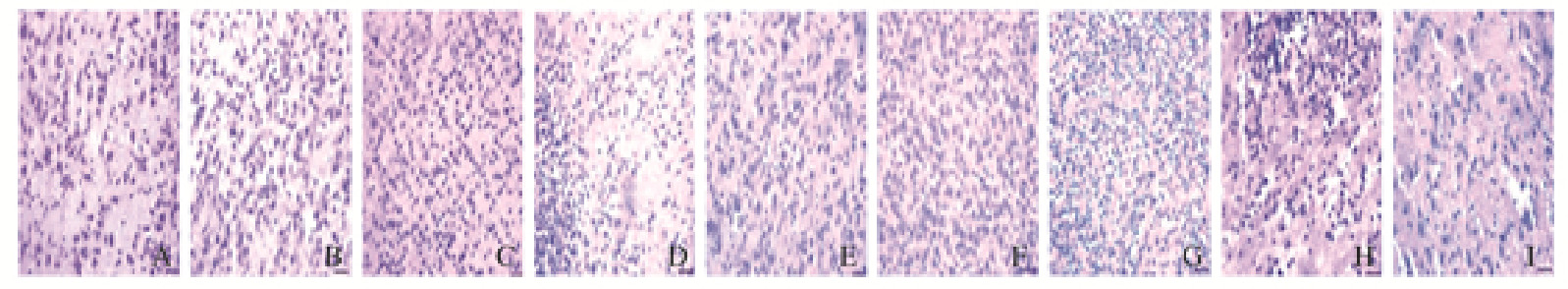
|
| A-C:Control group; D-F:Hyperoxia group; G-I:Hyperoxia+PGE-1 grouop; A, D, G:1 d; B, E, H:3 d; C, F, I:7 d. 图 1 不同时间点各组大鼠脑组织形态表现(HE,×400) Fig. 1 Morphology of brain tissue of rats in various groups at different time points (HE, ×400) |
|
|
经过TUNEL染色,第1、3和7天对照组大鼠脑组织细胞以大脑皮层细胞为主,凋亡细胞少见,胞核呈均质蓝色;同一时间高氧组大鼠脑组织中胞核呈亮白色,为阳性凋亡细胞;与高氧组比较,同一时间高氧+PGE-1组大鼠凋亡细胞数明显减少,见图 2(插页二)。同一时间高氧组大鼠细胞凋亡指数高于对照组(t=82.48,t=240.21,t=215.96, P<0.05);而高氧+PGE-1组大鼠凋亡指数低于高氧组(t=15.56,t=86.27,t=22.70, P<0.05)。见表 3。

|
| A-C:Control group; D-F:Hyperoxia group; G-I:Hyperoxia+PGE-1 grouop; A, D, G:1 d; B, E, H:3 d; C, F, I:7 d. 图 2 不同时间点各组大鼠脑细胞凋亡情况(TUNE,×400) Fig. 2 Apoptosis of brain cells of rats in various groups at different time points(TUNEL, ×400) |
|
|
| (n=10, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | Apoptotic index | ||||||||||||||||||||||||||||
| (t/d)1 | 3 | 7 | |||||||||||||||||||||||||||
| Control | 5.86±0.08 | 5.55±0.24 | 5.26±0.20 | ||||||||||||||||||||||||||
| Hyperoxia | 9.20±0.10* | 25.30±0.10* | 35.80±0.40* | ||||||||||||||||||||||||||
| Hyperoxia+ PGE-1 | 8.10±0.20△ | 19.20±0.20△ | 31.74±0.40△ | ||||||||||||||||||||||||||
| * P<0.05 compared with control group;△ P<0.05 compared with hyperoxia group. | |||||||||||||||||||||||||||||
第1、3和7天,与对照组比较,高氧组大鼠脑组织中p-JNK和JNK表达量增加(t=76.52, P<0.05);与同一时间高氧组比较,高氧+ PGE-1组大鼠脑组织中p-JNK和JNK表达量明显降低(t=23.1,P<0.05)。见图 3。
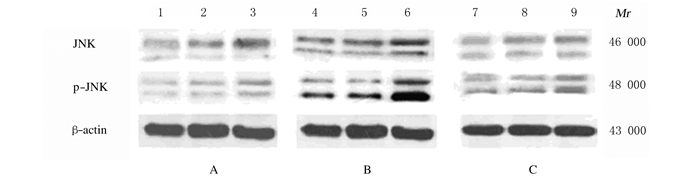
|
| A: Control group; B: Hyperoxia group; C:Hyperoxia+PGE-1 group.Lane 1, 4, 7:1 d; Lane 2, 5, 8:3 d; Lane 3, 6, 9:7 d. 图 3 不同时间点各组大鼠脑组织中p-JNK和JNK表达电泳图 Fig. 3 Electrophoregram of expressions of p-JNK and JNK in brain tissue of rats in various groups at different time points |
|
|
氧疗是救治新生儿尤其是早产儿过程中的重要措施,有研究者[7-8]认为:早产儿高氧暴露可能为脑性瘫痪和运动神经功能障碍的原因之一。有研究[9, 13]表明:生后1周新生大鼠相当于早产出生的新生儿。本研究通过构建高氧诱导新生大鼠脑损伤模型模拟新生儿高氧暴露后的脑损伤。在本实验中,高氧组新生大鼠脑组织病理切片呈现典型的脑损伤改变,随着高氧暴露时间延长,可出现神经元核固缩、深染和排列紊乱等表现,与部分研究结果[6, 10]一致,证实造模成功。
研究[11]表明:高氧脑损伤的重要原因之一可能为细胞凋亡。ESR是细胞凋亡的启动途径。ESR诱导细胞凋亡与多系统疾病具有相关性,如与神经退行性变、糖尿病、自身免疫性疾病和脑血管病等有关联[12]。ESR通过Caspase-12、CHOP和JNK等信号通路诱导细胞凋亡。研究[13-14]表明:Caspase-12和CHOP信号通路参与高氧脑损伤。本研究主要探讨JNK通路在高氧脑损伤中的变化及PGE-1的保护作用。
PGE-1为一种具有药理作用的天然活动物质,通常在细胞中均能内源性合成,在局部发挥生物学作用,并被迅速灭活,具有扩张血管、抗炎、保护血管内皮细胞和抗血小板凝集等作用[15],已被临床广泛应用于以心血管系统为主的多个领域。本研究中,同一时间高氧和高氧+ PGE-1组新生大鼠体质量较对照组明显降低,表明高氧环境会影响新生大鼠体质量增长,影响生长发育,导致发育迟缓,考虑高氧可加速机体新陈代谢率[16],引起热量吸收相对不足。同一时间,高氧+ PGE-1组大鼠体质量高于高氧组,表明PGE-1对高氧暴露环境中新生大鼠发育迟缓有一定干预作用。同一时间,高氧组大鼠脑组织含水量高于对照组,表明高氧暴露可导致脑组织细胞水肿;同时间,高氧+ PGE-1组大鼠脑组织质量低于高氧组,表明PGE-1可减轻脑组织水肿,考虑可能机制为高氧状态下,脑血管易发生收缩,导致脑血流量不足;PGE-1具有抗炎、扩张血管作用,可使脑血流增加,并减轻脑水肿,与杨山等[13]的研究结果一致。本研究中,高氧组新生大鼠脑组织病理切片出现皮层细胞形态学改变,随着高氧暴露时间延长,脑组织损伤加重,出现典型脑损伤病理学改变,如神经元核固缩、深染和排列紊乱,甚至神经元坏死脱失,神经细胞凋亡指数亦明显升高。研究[17]表明:高氧不但能减少神经营养因子的表达,而且还能够增加致炎细胞因子,进而加速了血管内皮细胞以及神经细胞的凋亡,与本研究结果一致。高氧+PGE-1组大鼠脑组织细胞损伤程度和细胞凋亡指数与同时间高氧组比较均降低,表明PGE-1对高氧诱导大鼠脑损伤具有一定保护作用。
JNK为哺乳动物细胞促分类原活化蛋白酶(MAOK)家族的一员[18],可通过钙调节蛋白触发Caspase和线粒体途径诱导细胞凋亡[19]。同时,还能上调Caspase-3、CHOP和Bcl-2家族中促凋亡因子p53及Bax等凋亡基因的表达,并且能下调Bcl-xl及Bcl-2等抗凋亡因子的表达[20-21],因此,JNK在诱导细胞凋亡中发挥重要作用。本研究中JNK蛋白表达量为NK1、JNK2和JNK3的总表达量,高氧组大鼠脑组织中p-JNK和JNK表达量较对照组明显增加,提示p-JNK及JNK表达量均增加,表明JNK通路被激活,蛋白磷酸化为具有更高活性的p-JNK,从而促进神经细胞发生凋亡,加重发育不完善脑组织的损伤,表明JNK信号通路参与了高氧诱发的脑损伤。高氧+ PGE-1组大鼠脑组织中p-JNK和JNK表达量均低于高氧组,表明PGE-1可下调ESR相关的p-JNK和JNK表达量。
综上所述,PGE-1对高氧诱导新生大鼠脑损伤具有一定保护作用,表现在可减轻发育迟缓、减轻脑水肿、减轻大脑皮层细胞损伤程度和降低神经细胞凋亡指数,其发挥保护作用机制之一可能通过下调ESR相关的p-JNK和JNK蛋白表达实现的。
| [1] |
吴瑕, 柳国胜, 罗瑶, 等. 细胞粘附因子-1在新生大鼠高氧肺损伤模型肺组织的表达及意义[J]. 中国新生儿科杂志, 2012, 27(2): 124-127. DOI:10.3969/j.issn.1673-6710.2012.02.017 |
| [2] |
张喆, 曹大伟, 张新日. 高氧肺损伤机制研究进展[J]. 国际呼吸杂志, 2018, 38(6): 461-464. DOI:10.3760/cma.j.issn.1673-436X.2018.06.014 |
| [3] |
郑潇玥, 陈晓. 氧化应激在重症新生儿高氧肺损伤中的作用研究进展[J]. 南昌大学学报:医学版, 2016, 56(1): 92-95. |
| [4] |
NICU早产儿用氧及ROP防治现状调查组. 16家三甲医院新生儿重症监护病房早产儿用氧及早产儿视网膜病变防治现状调查[J]. 中华儿科杂志, 2012, 50(3): 167-171. DOI:10.3760/cma.j.issn.0578-1310.2012.03.003 |
| [5] |
GOREN B, CAKIR A, SEVINC C, et al. Uridine treatment protects against neonatal damage and long-term cognitive deficits caused by hyperoxia[J]. Brain Res, 2017, 1676(12): 57-68. |
| [6] |
宋朝敏, 王程毅, 陈涵强, 等. 新生大鼠高氧脑损伤的评价研究[J]. 中国新生儿杂志, 2014, 29(1): 54-57. |
| [7] |
ALLI M P, KONTIS D. White matter and congnition in adults who were born preterm[J]. PLoS One, 2011, 6(10): e24525. DOI:10.1371/journal.pone.0024525 |
| [8] |
VOLPE J J. Brain injury in premature infants:a complex amalgam of destructive and developmental disturbances[J]. Lancet Neurol, 2009, 8(1): 110-124. |
| [9] |
SEMPLE B D, BLOMGREN K, GIMLIN K, et al. Brain development in rodents and humans:Identifying benchmarks of maturation and vulnerability to injury across species[J]. Prog Neurobiol, 2013, 106-107(7/8): 1-16. |
| [10] |
杨磊. 胰岛素样生长因子-1在新生大鼠高氧脑损伤的研究进展[J]. 吉林医学, 2016, 37(3): 700-702. DOI:10.3969/j.issn.1004-0412.2016.03.098 |
| [11] |
VOTTIER G, PHAM H, PANSIOT J, et al. Deleterious effect of hyperoxia at birth on white matter damage in the newborn rat[J]. Dev Neurosci, 2011, 33(3-4): 261-269. DOI:10.1159/000327245 |
| [12] |
KIM I, XU W, REED J C, et al. Cell death and endoplasmic reticulum Stress:disease relevance and therapeutic opportunities[J]. Nat Rev Drug Discov, 2008, 7(12): 1013-1030. DOI:10.1038/nrd2755 |
| [13] |
杨山, 张有辰, 李慧文, 等. 前列腺素E1对高氧诱导新生大鼠脑损伤额保护作用[J]. 中国当代儿科杂志, 2018, 20(3): 230-235. |
| [14] |
黄久浪, 韩颖, 崔晨, 等. 内质网应激在高氧导致新生小鼠脑白质损伤中的调控作用[J]. 中国新生儿在职, 2016, 31(3): 206-211. |
| [15] |
刘春云, 刘永平, 龚享文. 前列地尔药理学研究进展及在呼吸系统疾病中的应用[J]. 中国新药与临床杂志, 2012, 31(11): 648-651. |
| [16] |
MATSUMOTO A, OKIURA T, MORIMATSU F, et al. Effects ofhyperbaric exposure with high oxygen concentration on thephysical activity of developing rats[J]. Dev Neurosci, 2007, 29(6): 452-459. DOI:10.1159/000097319 |
| [17] |
GERSTNER B, DESILVA TM, GENZ K, et al. Hyperoxia CausesMaturation-dependent cell death in the developing white matter[J]. J Neurosci, 2008, 28(5): 1236-1245. DOI:10.1523/JNEUROSCI.3213-07.2008 |
| [18] |
BHARDWAJ M, PAUL S, JAKHAR R, et al. Potential role of vitexin in alleviating heat stress-induced cytotoxicity:Regulatory effect of Hsp90 on ER stress-mediated autophagy[J]. Life Sci, 2015, 142(12): 36-48. |
| [19] |
URANO F, WANG X., BERTOLOTTI A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE 1[J]. Science, 2000, 287(5453): 664-666. DOI:10.1126/science.287.5453.664 |
| [20] |
SHIGEMI Z, MANABE K, HARA N, et al. Methyseleninic acid and sodium selenite induce severe ER stress and subsequent apoptosis through UPR activation in PEL cells[J]. Chem Biol Interact, 2017, 266(3): 28-37. |
| [21] |
王一超, 刘俊杰, 刘海宁, 等. Toll样受体4在蛛网膜下腔出血大鼠早期脑损伤中的作用及对海马区神经元自噬的影响[J/OL].西安交通大学学报: 医学版, 2019[2019-11-18]. http://kns.cnki.net/kcms/detail/61.1399.r.20190930.1115.006.html.
|
 2019, Vol. 45
2019, Vol. 45


