扩展功能
文章信息
- 李文静, 田松波, 程冬梅, 刘从娜, 崔占琴
- LI Wenjing, TIAN Songbo, CHENG Dongmei, LIU Congna, CUI Zhanqin
- 神经生长因子对比格犬年轻恒牙牙髓组织再生的促进作用
- Promotion effect of nerve growth factor on regeneration of young permanent dental pulp tissue in Beagle dogs
- 吉林大学学报(医学版), 2019, 45(05): 1075-1079
- Journal of Jilin University (Medicine Edition), 2019, 45(05): 1075-1079
- 10.13481/j.1671-587x.20190518
-
文章历史
- 收稿日期: 2018-11-17
2. 河北医科大学第二医院口腔正畸科, 河北 石家庄 050000
2. Department of Orthodontics, Second Hospital, Heibei Medicial University, Shijiazhuang 050000, China
年轻恒牙由于发育畸形、龋齿和外伤等容易引起牙髓感染,进一步发展为牙根尖周炎,致龋菌代谢产酸使牙髓暴露感染,导致牙髓根尖周炎的发生[1]。根尖周炎常规治疗方法为根管治疗术,治疗后可导致牙齿抗折能力不同程度地下降,牙髓再生作为一种替代疗法受到研究者的关注[2]。神经生长因子(nerve growth factor,NGF)具有影响免疫细胞活性、调节免疫系统功能[3]、修复创伤组织[4]、促进牙髓细胞增殖和分化及修复损伤牙髓组织等作用,在多种疾病的治疗中具有重要作用[5-6]。在牙胚来源的外胚间充质干细胞中NGF呈节律性变化,NGF被证明是牙齿领域的生物节律调控因子[7];NGF对种植牙周围损伤神经修复和再生具有促进作用[8],但目前尚未发现NGF对根尖周炎牙髓组织增生影响的研究。本研究采用NGF对比格犬根尖周炎进行治疗,观察其对牙髓组织增生的影响,探讨NGF在根尖周炎治疗中的作用。
1 材料与方法 1.1 实验动物、主要试剂和仪器6只健康比格犬,雌雄各半,纯种、清洁级、5月龄,购自北京芳元缘养殖中心,动物许可证号:SYXK(京)2014-0039。纳入标准:X线检查证实根尖闭合的上颌前牙和下颌前牙。排除标准:牙齿松动Ⅱ度及以上者,牙根暴露,牙周探诊深度>2 mm,牙冠存在隐裂。根据纳入及排除标准,将6只比格犬共12颗牙齿纳入研究(每只犬纳入2颗牙齿)。注射用鼠NGF(武汉海特生物制药股份有限公司,国药准字:S20060051,批号:20110608),BD水凝胶支架(美国Corning公司),玻璃离子三氧化物聚合体MTA(美国Dentsply公司),戊巴比妥钠(德国Merck公司),EDTA凝胶(美国PULPDENT公司)。便携式牙片机(中国天杰公司),光学显微镜及照相系统(日本Olympus公司),Woodpex Ⅲ长度测量仪(桂林市啄木鸟医疗器械有限公司),体视显微镜(江西凤凰光学控股有限公司)。
1.2 实验动物分组和动物模型的建立将6只比格犬12颗牙齿分为对照组、水凝胶组和水凝胶复合NGF组,每组2只犬4颗牙。对照组不予处理;水凝胶组建立根尖周炎模型,给予单纯水凝胶处理;水凝胶复合NGF组建立根尖周炎模型,给予NGF复合水凝胶处理。
水凝胶组和水凝胶复合NGF组建立比格犬根尖周炎模型[9]:将实验比格犬用戊巴比妥腹腔注射麻醉成功后,2%利多卡因0.2 g局部麻醉上下颌前牙区,实验牙开髓,拔髓,疏通牙根管,将比格犬自体牙菌斑溶于生理盐水中,形成菌斑液,棉捻蘸取菌斑液封入根管内。1个月后进行X线检查观察牙根尖病变情况,经2名医师鉴定证实,与术前比较,牙根尖出现低密度影确定根尖周炎模型建立成功。
1.3 根管封药实验牙根尖周炎模型建立成功后,戊巴比妥腹腔麻醉比格犬,取出封入牙根管的棉捻,用根管锉疏通牙根管,结合X线采用Woodpex Ⅲ根管长度测量仪确定工作长度,用1%次氯酸钠(NaClO)冲洗牙根管,干燥后牙根管内倒入环丙沙星、甲硝唑和米诺环素等量混合的三联抗生素,用硫酸锌严密封闭冠部。制备NGF复合水凝胶[7]:将NGF用5%海藻糖溶液溶解,稀释浓度为1 000 μg·L-1,90 μL水凝胶溶液中加入10 μL NGF制成100 μg·L-1的NGF复合水凝胶。
1.4 牙髓治疗根管封药后1个月,X线检查观察到根尖周低密度影消失,戊巴比妥腹腔麻醉比格犬,洗必泰棉球消毒手术区域,上橡皮障,取出暂封膏,用NaClO和生理盐水冲洗根管,冲出抗生素糊剂,无菌纸拭干根管。水凝胶复合NGF组牙根管内植入NGF复合水凝胶(浓度为100 μg·L-1)至NGF复合水凝胶溢出根管口;水凝胶组牙根管内植入等量单纯水凝胶,待凝固后,将表层凝固的凝胶去除,建立充填窝洞,剥离离子和MTA封填窝洞;对照组不处理。
1.5 组织学检测植入后3个月,戊巴比妥钠腹腔麻醉比格犬,动脉灌注固定,取出样本,甲醛固定,EDTA脱钙,X线检查证实脱钙完成后使用脱水机脱水,石蜡包埋,沿牙根长轴纵向将每个标本切成厚度为4 μm连续切片。HE染色:将石蜡切片烤箱烤片2 h,脱蜡,梯度酒精复水,苏木素染色,碳酸锂返蓝,伊红染色,梯度酒精脱水,干燥箱干燥,二甲苯透明、中性树脂封片,显微镜下观察HE染色情况。
1.6 根管再生组织面积百分比的测定显微镜下拍摄每个标本切片HE染色全景图像,使用C6显微刻度尺制作标定,使用WoodpexⅢ长度测量仪测定切片根尖顶点到根管内再生组织面最高处之间的距离(以根管长轴垂直平行生成网格线以避免测量误差);使用面积测量工具圈定根管内再生组织和根管腔,根据软件自动测量再生组织面积和根管腔面积,计算根管再生组织的面积百分比(再生组织面积/根管腔面积×100%)。
1.7 统计学分析采用SPSS 20.0软件进行数据分析处理。再生组织长入深度和再生组织面积百分比符合正态分布,以x±s表示,2组间样本均数比较采用两独立样本t检验。检验水准为α=0.05。
2 结果 2.1 比格犬恒牙根尖周炎模型的建立比格犬年轻恒牙经过开髓、根管内封菌斑捻1个月后,检查可见牙龈红肿,牙齿Ⅱ度松动,牙周溢脓。X线检查可见牙根分歧下和牙根尖周出现低密度影,提示比格犬年轻恒牙根尖周炎模型建立成功。见图 1。
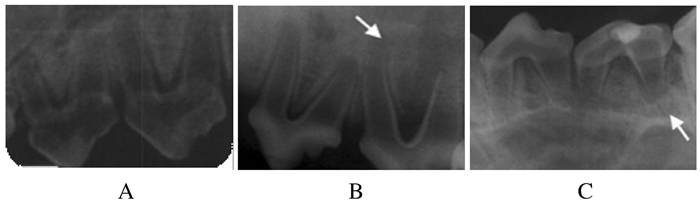
|
| A: Control group; B: Hydrogel group; C:Hydrogel combined with NGF group. The arrows referred to the low density shadow. 图 1 比格犬年轻恒牙根尖周炎模型X线检查结果 Fig. 1 X-ray examination results of young permanent teeth perapical perioditis models of Beagle dogs |
|
|
对照组牙根管外壁的牙骨质和内壁的牙本质沉积使牙根管壁增厚,牙骨质包围根尖部;根管内牙髓组织正常,由成纤维细胞、成牙本质细胞和血管组成,牙本质细胞和牙本质壁相连接(图 2A,见插页五)。水凝胶组牙根尖部无新形成硬组织,根尖周出现纤维修复,根管局部内吸收,吸收部位见牙骨质样外观的新形成组织(图 2B,见插页五)。水凝胶复合NGF组牙根管内有新组织形成,牙根管内邻近牙本质壁有新形成的硬组织,新形成的硬组织使根管壁增厚,与牙骨质相似,牙骨质细胞样细胞和牙骨质基质陷窝明显可见;根管内见游离的牙骨质样组织,与周围硬组织不相连,牙根管内新形成的软组织主要为血管和成纤维细胞(图 2C,见插页五)。
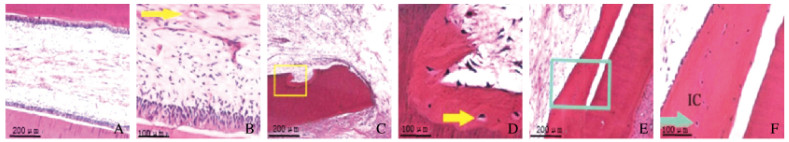
|
| A, B: Control group; C, D:Hydrogel group; E, F:Hydrogel combined with NGF group; A, C, E: Pulp tissue in root canal; B, D:Enlarged detail of pulp of Beagle dogs in tissue (The arrow refferred to the blood vessel); F: Enlarged detail of A-frame(The arrow showed the deposition of cementum-like cells in hard tissue). 图 2 各组比格犬恒牙牙髓组织形态表现 Fig. 2 Morphology of permanent teeth pulp tissue of Beagle dogs in various groups |
|
|
水凝胶复合NGF组牙根管再生组织长入深度和再生组织面积百分比均高于水凝胶组(P < 0.05)。见表 1。
| (n=4, x±s) | ||
| Group | Depth of regenerated tissue(l/mm) | Percentage area of regenerated tissue(η/%) |
| Hydrogel | 0.27±0.05 | 2.13±0.42 |
| Hydrogel combined with NGF | 3.12±0.07* | 22.54±0.53* |
| * P < 0.01 compared with hydrogel group. | ||
龋病较高的患病率使根尖周炎成为牙体牙髓科最常见的疾病之一,常规的根管治疗术后根管壁被切削,由于失去牙髓营养使牙齿抗折能力下降[11-12]。牙髓由神经支配,具有高度血管化的结缔组织,可为牙体提供营养,使牙本质保持活力;可以使形成牙本质细胞分泌牙本质基质,形成牙本质;牙髓具有再生能力[13-14]。但由于牙髓的解剖结构以及牙本质、牙髓细胞的特性限制了牙髓的修复再生能力[15]。因此促进牙髓再生是替代常规根管治疗术的一种有效疗法[16],受到广大学者关注。
细胞因子在参与牙髓再生过程中可调节牙髓细胞增殖、分化、凋亡和迁移等。NGF在神经的发育、生长、存活和分化等方面具有重要作用[17-19];NGF可促进血管平滑肌细胞和内皮细胞等的分化及迁移,动员内皮祖细胞,促进内皮祖细胞活化,形成新生血管,也可与血管内皮生长因子相互作用促进内皮细胞迁移、分化和增生,完成血管生成过程[20-22];NGF参与牙齿发育的调控,在牙齿受到损伤或炎症感染后牙髓组织中NGF表达水平升高,牙髓局部NGF浓度的升高不仅可以促进牙髓损伤处感觉神经纤维再生,还可促进P物质和降钙素基因相关肽等神经肽水平升高,导致痛觉过敏的产生,升高的降钙素基因相关肽可明显增加碱性磷酸酶活性,刺激牙髓细胞分化为牙本质,促进矿化形成;NGF本身也可促进人牙髓细胞的增殖和向成牙本质细胞分化[23-24]。
基于NGF的上述特点,考虑其可能促进根尖周炎牙髓组织的再生。本研究建立比格犬根尖周炎模型,根管内给予NGF复合水凝胶治疗,结果显示:NGF治疗后牙根管内有新形成的硬组织和软组织,新形成的硬组织中可见牙骨质细胞样细胞和牙骨质基质陷窝,根管内可见游离的牙骨质样组织,新形成的软组织主要为血管和成纤维细胞;而根管内给予单纯水凝胶的水凝胶组牙根尖部无新形成硬组织,根尖周出现纤维修复;水凝胶复合NGF组牙根管内再生组织长入深度和再生组织面积百分比均高于水凝胶组。本研究结果表明:NGF可促进根尖周炎牙髓组织再生,水凝胶复合NGF组牙根管内可见新形成的硬组织,可能是NGF促进牙髓细胞向成牙本质细胞分化、促进矿化形成的结果;水凝胶复合NGF组牙根管内新生软组织主要为血管和成纤维细胞,可能与NGF可促进牙髓细胞增殖和促进新生血管生成等有关。
综上所述,NGF可促进根尖周炎牙髓组织的再生,本研究结果为根尖周炎的治疗提供了新的思路。
| [1] | LIN JC, ZENG Q, WEI X, et al. Regenerative endodontics versus apexification in immature permanent teeth withapical periodontitis:A prospective randomized controlled study[J]. J Endod, 2017, 43(11): 1821–1827. DOI:10.1016/j.joen.2017.06.023 |
| [2] | PALMA P J, RAMOS J C, MARTINS J B, et al. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis[J]. J Endod, 2017, 43(8): 1279–1287. DOI:10.1016/j.joen.2017.03.005 |
| [3] | SURUGA K, KADOKURA K, SEKINO Y, et al. Effects of comb tooth cap medicinal mushroom, hericium ramosum (higher basidiomycetes) mycelia on DPPH radical scavenging activity and nerve growth factor synthesis[J]. Int J Med Mushrooms, 2015, 17(4): 331–338. DOI:10.1615/IntJMedMushrooms.v17.i4.20 |
| [4] | EAP S, BÉCAVIN T, KELLER L, et al. Nanofibers implant functionalized by neural growth factor as a strategy to innervate a bioengineered tooth[J]. Adv Healthc Mater, 2014, 3(3): 3863–3891. |
| [5] | ZHAO J, GUO Y, LAN A, et al. The effect of amino plasma-enhanced chemical vapor deposition-treated titanium surface on Schwann cells[J]. J Biomed Mater Res A, 2018, 106(1): 265–271. DOI:10.1002/jbm.a.36167 |
| [6] | PARK S, CHOI Y, KWAK G, et al. Application of differentiated human tonsil-derived stem cells to trembler-J mice[J]. Muscle Nerve, 2018, 57(3): 478–486. DOI:10.1002/mus.25763 |
| [7] | 杨琨, 李骏, 丰奇昊, 等. 大鼠牙胚来源外胚间充质干细胞中p75NTR时钟节律性表达[J]. 现代医药卫生, 2018, 34(20): 3105–3108, 3111. DOI:10.3969/j.issn.1009-5519.2018.20.001 |
| [8] | 冼海瑜, 郭竹玲. 神经生长因子促种植体周围神经再生的研究[J]. 全科口腔医学电子杂志, 2018, 5(24): 25–26. DOI:10.3969/j.issn.2095-7882.2018.24.013 |
| [9] | 陈敏, 刘雪梅, 包志凡, 等. 犬年轻恒牙根尖周炎和再生性牙髓治疗后组织学观察[J]. 中国实用口腔科杂志, 2015, 8(9): 532–535. |
| [10] | KIM J Y, XIN X J, MOIOLI E K, et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing[J]. Tissue Eng Part A, 2010, 16(10): 3023–3031. DOI:10.1089/ten.tea.2010.0181 |
| [11] | BERLIN-BRONER Y, FEBBRAIO M, LEVIN L. Apical periodontitis and atherosclerosis:Is there a link review of the literature and potential mechanism of linkage[J]. Quintessence Int, 2017, 48(7): 527–534. |
| [12] | ALTAII M, CATHRO P, BROBERG M, et al. Endodontic regeneration and tooth revitalization in immature infected sheep teeth[J]. Int Endod J, 2017, 50(5): 480–491. DOI:10.1111/iej.12645 |
| [13] | ASGARY S, FAZLYAB M, NOSRAT A. Regenerative endodontic treatment versus apical plug in immature teeth:Three-year follow-up[J]. J Clin Pediatr Dent, 2016, 40(5): 356–360. DOI:10.17796/1053-4628-40.5.356 |
| [14] | 杨懋彬, 曾倩. 牙髓根尖周病的治疗新方法:再生牙髓病学之路[J]. 口腔疾病防治, 2017, 25(2): 74–79. |
| [15] | GAVIÑO ORDUÑA J F, CAVIEDES-BUCHELI J, MANZANARES CÉSPEDES M C, et al. Use of platelet-rich plasma in endodontic procedures in adults:regeneration or repair a report of 3 cases with 5 years of follow-up[J]. J Endod, 2017, 43(8): 1294–1301. DOI:10.1016/j.joen.2017.04.010 |
| [16] | 雷期音, 陈柯. 年轻恒牙牙髓再生的临床应用进展[J]. 国际口腔医学杂志, 2017, 44(3): 267–272. |
| [17] | NORMAN B H, MCDERMOTT J S. Targeting the nerve growth factor(NGF) pathway in drug discovery. potential applications to new therapies for chronic pain[J]. J Med Chem, 2017, 60(1): 66–88. |
| [18] | MAHDEE A, EASTHAM J, WHITWORTH JM, et al. Evidence for changing nerve growth factor signalling mechanisms during development, maturation and ageing in the rat molar pulp[J]. Int Endod J, 2019, 52(2): 211–222. DOI:10.1111/iej.12997 |
| [19] | ROCCO M L, SOLIGO M, MANNI L, et al. Nerve growth factor:Early studies and recent clinical trials[J]. Curr Neuropharmacol, 2018, 16(10): 1455–1465. DOI:10.2174/1570159X16666180412092859 |
| [20] | MOATTARI M, KOUCHESFEHANI H M, KAKA G, et al. Evaluation of nerve growth factor (NGF) treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury[J]. J Craniomaxillofac Surg, 2018, 46(6): 898–904. DOI:10.1016/j.jcms.2018.03.015 |
| [21] | SONE Y, TAKATORI S, OCHI E, et al. Nerve growth factor facilitates the innervation of perivascular nerves in tumor-derived neovasculature in the mouse cornea[J]. Pharmacology, 2017, 99(1/2): 57–66. |
| [22] | AHLUWALIA A, JONES M K, BRZOZOWSKI T, et al. Nerve growth factor is critical requirement for in vitro angiogenesis in gastric endothelial cells[J]. Am J Physiol Gastrointest Liver Physiol, 2016, 311(5): G981–G987. DOI:10.1152/ajpgi.00334.2016 |
| [23] | MITSIADIS T A, MAGLOIRE H, PAGELLA P. Nerve growth factor signalling in pathology and regeneration of human teeth[J]. Sci Rep, 2017, 7(1): 1327–1327. DOI:10.1038/s41598-017-01455-3 |
| [24] | LI J Y, PARADA C, CHAI Y. Cellular and molecular mechanisms of tooth root development[J]. Development, 2017, 144(3): 374–384. DOI:10.1242/dev.137216 |
 2019, Vol. 45
2019, Vol. 45


