扩展功能
文章信息
- 刘俊杰, 杜鹃, 杨雅菊, 王雪, 孙玉婷, 刘瑶, 赵雅宁, 李建民
- LIU Junjie, DU Juan, YANG Yaju, WANG Xue, SUN Yuting, LIU Yao, ZHAO Yaning, LI Jianmin
- 头孢曲松对蛛网膜下腔出血大鼠海马神经细胞的保护作用及其机制
- Protective effect of ceftriaxone on hippocampal neurons in subarachnoid hemorrhage rats and its mechanism
- 吉林大学学报(医学版), 2019, 45(05): 1069-1074
- Journal of Jilin University (Medicine Edition), 2019, 45(05): 1069-1074
- 10.13481/j.1671-587x.20190517
-
文章历史
- 收稿日期: 2018-12-21
2. 华北理工大学附属医院神经外科, 河北 唐山 063000;
3. 华北理工大学护理与康复学院护理学系, 河北 唐山 063000
2. Department of Neurosurgery, Affiliated Hospital, North China University of Science and Technology, Tangshan 063000, China;
3. Department of Nursing, College of Nursing and Rehabilitation, North China University of Science and Technology, Tangshan 063000, China
蛛网膜下腔出血(subarachnoid hemorrhage, SAH)是常见的中枢神经系统疾病,严重威胁人类健康,世界卫生组织(WHO)报道,SAH的患病率以每年22.5/10万的速度增长[1]。由于SAH的发病率和死亡率高,切实可行的治疗手段有限[2],因此探讨SAH后的神经损伤机制,寻找新型治疗药物及调控靶点成为目前研究的重点。研究[3]表明:早期脑损伤(early brain injury, EBI)在SAH的神经损伤中起主要作用,其涉及炎症、氧化应激、血管痉挛和细胞死亡等病理过程。头孢曲松(ceftriaxone, CEF)是一种β-内酰胺类抗生素,近年来研究[4]证实:CEF对脑缺血大鼠具有神经保护作用,可以上调谷氨酸转运体蛋白的表达,减轻兴奋性氨基酸的毒性作用;也有研究[5-6]证实:CEF可以减轻SAH大鼠神经细胞的炎症反应,改善神经功能。然而,CEF对SAH大鼠的神经保护机制尚未完全阐明。本研究拟建立大鼠SAH模型,观察CEF对SAH大鼠EBI损伤的治疗作用及海马区神经细胞自噬及凋亡的影响,以期探讨CEF对SAH导致的神经损伤的保护作用及其机制。
1 材料与方法 1.1 实验动物及分组清洁级雄性SD大鼠48只,体质量350~450 g,由北京维通利华实验动物科技有限公司提供,动物许可证号:SYXK(京)2014-0002。将48只大鼠随机分为假手术组、蛛网膜下腔出血模型组(SAH组)、3-甲基腺嘌呤干预组(3-MA组)和头孢曲松治疗组(CEF组),每组12只。
1.2 主要试剂和仪器CEF(批号:0080418,齐鲁制药有限公司),3-MA溶液(M9281-100MG,美国Sigma公司),多克隆Beclin-1、LC3-Ⅱ抗体和TUNEL试剂盒(美国Santa Cruz公司),二抗及其辅助用品(博奥森生物技术有限公司)。切片机(Leica RM2235型,德国LEICA公司),Motic-6.0图像采集及分析系统和Olympus BX53型光学显微镜(日本Olympus公司)。
1.3 实验动物模型制备及给药方法参照BARRY等[7]文献报道,采用改良血管内穿刺法制备大鼠SAH模型:大鼠称体质量并经腹腔注射10%水合氯醛3.5 mL·kg-1麻醉,仰卧固定,颈部正中切口,分离右侧颈总、颈外和颈内动脉,结扎、离断颈外动脉。此时将颈外动脉残端处丝线的松结拉紧,除去颈内动脉的动脉夹,继续将穿刺线插入,至感到有阻力稍用力再插入1.0~1.5 mm后有落空感即可抽出并除去微动脉夹。整个手术过程保持大鼠肛温(37±1)℃。假手术组手术操作与SAH组相同,但仅穿刺不刺破血管。造模成功指标:术中大鼠出现呼吸急促,心率加快;麻醉复苏后精神萎靡、嗜睡、畏光、易惊厥、自洁性差;处死后取脑可见蛛网膜下腔散在分布大量血凝块。实验中剔除死亡大鼠,严格按实验条件进行补充分组(造模后SAH模型组死亡1只;3-MA组死亡2只,不符合评分标准1只;CEF组死亡2只,不符合评分标准2只)。大鼠术后即可通过腹腔注射法注射相应药品,3-MA组大鼠注射3-MA,给药剂量为15 mg·kg-1;CEF组大鼠注射CEF,给药剂量为500 mg·kg-1。
1.4 大鼠神经功能评分采用改良Garcia评分表[8],在SAH模型建立成功后相应时间点评价各组大鼠的自发活动、自发四肢运动、前肢伸展活动、攀登反应、双侧躯干触碰反应和触须反应,各部分得分相加得出总的神经功能评分,其评分越低,则提示神经系统损伤越严重,评分越高,则神经损伤越轻,最低评分为3分,最高评分为18分。
1.5 HE染色观察大鼠海马CA1区细胞死亡情况大鼠SAH模型制备成功后24 h,10%水合氯醛深度麻醉大鼠后打开胸腔,经心脏灌注生理盐水至大鼠肝脏发白,随后改用4%多聚甲醛灌注,断头取脑,置于甲醛溶液内保存。纵切取视交叉至大脑横裂区域脑组织,行常规石蜡包埋、切片(切片厚度5 μm)、HE染色,脱水封片。光镜下观察大鼠海马CA1区细胞的病理表现。每张切片取6个视野,Motic-6.0图像采集及分析系统记录高倍视野下坏死神经细胞数量并计算均值。
1.6 免疫组织化学法检测各组大鼠海马组织中Beclin-1和LC3-Ⅱ阳性细胞数行常规脱蜡水化,经过氧化氢避光封闭后使用胰酶抗原修复(胰酶1:4稀释),随后滴加Beclin-1多克隆抗体(1:200稀释)、LC3-Ⅱ多克隆抗体(1:300稀释)置于湿盒中4℃冰箱过夜;第二步滴加二抗置于37℃温箱孵育30 min,经PBS洗涤,行DAB显色、脱水、透明、封片。实验采用PBS代替一抗作为阴性对照。光学显微镜下观察大鼠海马CA1区细胞的阳性表达情况,阳性细胞呈棕褐色。镜下观察并摄片,每张切片在海马CA1区随机选取5个视野,计数Beclin-1和LC3-Ⅱ阳性细胞数。
1.7 Western blotting法检测各组大鼠海马组织中Beclin-1、LC3-Ⅱ和caspase蛋白表达水平取保存于-80℃的视交叉至大脑横裂区域脑组织,加入裂解液(脑组织体积:裂解液=1:4)置于匀浆机内充分研磨后,4℃、12 000 r·min-1离心20 min提取蛋白。使用BCA法进行海马蛋白定量,配制上样缓冲液,行电泳分离海马蛋白。随后将蛋白转移到硝酸纤维膜上,用10%脱脂牛奶封闭1 h,加Beclin-1(取样比例为1:1 000)、LC3-Ⅱ(取样比例为1:1 000)和caspase(取样比例为1:1500),4℃冰箱过夜。隔夜取出滴加二抗,37℃恒温孵育30 min。TBST洗涤后用显影液显影,曝光保存图片。内参为β-actin。采用Western blotting法测定其灰度值,并以β-actin条带作为参照物。目的蛋白表达水平=目的蛋白条带灰度值/β-actin条带灰度值。
1.8 TUNEL法检测细胞凋亡数切片进行常规脱蜡、预处理组织后,使用过氧化氢室温封闭10 min;PBS洗涤,随后滴加平衡缓冲液,风干多余液体立即滴加工作强度转移酶充分覆盖组织,并置于湿盒中温箱孵育1 h,使用工作强度停止/洗涤液停止反应;最后滴加抗地高辛氧化酶经室温孵育30 min后,滴加DAB显色、脱水、透明、封片。光学显微镜下TUNEL阳性产物主要位于细胞核。镜下观察并摄片,每张切片在海马CA1区随机选取5个视野,采用Image Pro Plus 6.0软件进行TUNEL阳性细胞计数,即为凋亡细胞数量。
1.9 统计学分析采用SPSS 19.0统计软件进行统计学分析。Garcia评分,坏死神经细胞数,Beclin-1和LC3-Ⅱ阳性细胞数及蛋白表达水平,凋亡细胞数,Caspase-3蛋白表达水平以x±s表示。多组间样本均数比较采用单因素方差分析,组间两两比较采用Dunnett-t检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠神经功能评分假手术组、SAH组、3-MA组和CEF组大鼠海马区神经元功能评分分别为(18.00±1.36)分、(4.00±0.57)分、(3.00±0.69)分和(6.00±1.58)分。与假手术组比较,SAH组大鼠Garcia评分降低(P < 0.05);与SAH组比较,3-MA组大鼠Garcia评分明显降低(P < 0.05),而CEF组大鼠Garcia评分明显升高(P < 0.05)。
2.2 各组大鼠海马区神经细胞形态及数量假手术组大鼠海马细胞形态规则、分层排列整齐,胞浆均匀,胞核界限清晰,核仁明显;SAH组大鼠海马细胞形态呈不规则锥形、交错排列,核膜裂解、核固缩和溶解,核仁消失;与SAH组比较,3-MA组大鼠海马驱神经细胞病理损伤及核固缩和核溶解加重;与SAH组比较,CEF组大鼠海马区神经细胞损伤明显减轻,核固缩、核碎裂和核溶解现象明显减轻,但较假手术组损伤严重。假手术组、SAH组、3-MA组和CEF组大鼠海马区坏死神经细胞数分别为(3.85±0.56)、(46.59±3.45)、(59.36±4.68)和(38.43±3.95)个;与SAH组比较,3-MA组大鼠海马区坏死神经细胞数增加(P < 0.05),CEF组大鼠海马区坏死神经细胞数减少(P < 0.05)。见图 1(插页四)。
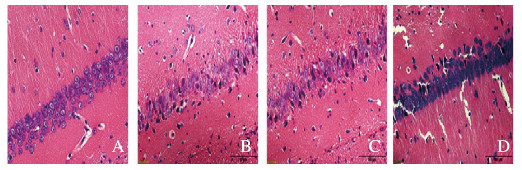
|
| A:Sham operation group; B:SAH group; C:3-MA group; D:CEF group. 图 1 各组大鼠海马区神经元形态表现(HE,×400) Fig. 1 Morphology of neurons in hippocampus of rats in various groups (HE, ×400) |
|
|
假手术组大鼠海马组织中散在分布少量Beclin-1和LC3-Ⅱ阳性细胞,Beclin-1和LC3-Ⅱ蛋白均为低水平表达;与假手术组比较,SAH组大鼠海马组织中Beclin-1和LC3-Ⅱ阳性细胞数明显增加(P < 0.05);与SAH组比较,3-MA组大鼠海马组织中Beclin-1和LC3-Ⅱ阳性细胞数明显减少,但仍高于假手术组(P < 0.05);与SAH组比较,CEF组大鼠海马组织中Beclin-1和LC3-Ⅱ阳性细胞数明显增加(P < 0.05)。见图 2(插页四)和表 1。

|
| A-D:Beclin-1;E-H:LC3-Ⅱ; A, E:Sham operation group; B, F:SAH group; C, G:3-MA group; D, H:CEF group. 图 2 各组大鼠海马组织中Beclin-1和LC3-Ⅱ表达情况(免疫组织化学,×400) Fig. 2 Expressions of Beclin-1 and LC3-Ⅱ in hippocampus tissue of rats in various groups (Immunohistochemistry, ×400) |
|
|
| (n=6, x±s) | ||
| Group | Number of positive cells | |
| Beclin-1 | LC3-Ⅱ | |
| Sham operation | 4.70±0.56 | 8.00±0.25 |
| SAH | 26.20±2.34* | 23.70±1.25* |
| 3-MA | 18.20±1.25△ | 14.90±1.23△ |
| CEF | 30.80±2.26△ | 33.60±4.32△ |
| * P < 0.05 vs sham operation group;△P < 0.05 vs SAH group. | ||
假手术组大鼠海马区只有极少凋亡细胞;与假手术组比较,SAH组大鼠海马区凋亡细胞数明显增多(P < 0.05);与SAH组比较,CEF组大鼠海马区凋亡细胞明显减少(P < 0.05),而3-MA组大鼠海马区凋亡细胞数明显增加(P < 0.05)。Western blotting法检测结果显示:与SAH组比较,CEF组大鼠海马组织中Caspase-3蛋白表达水平明显降低(P < 0.05),而3-MA组Caspase-3表达水平明显升高(P < 0.05)。见图 3(插页五)和图 4~5。

|
| 图 3 各组大鼠海马组织中神经细胞形态表现(TUNEL, ×400) Fig. 3 Morphology of nerve cells in hippocampus tissue of rats in various groups(TUNEL, ×400) |
|
|
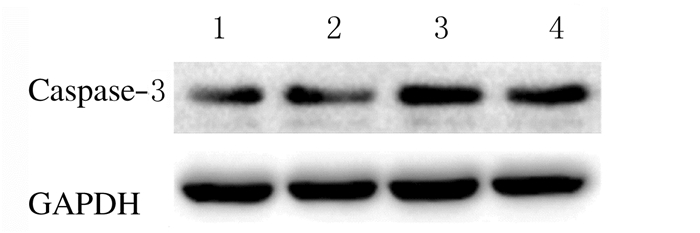
|
| Lane 1:Sham operation group; Lane 2:SAH group; Lane 3:3-MA group; Lane 4:CEF group. 图 4 各组大鼠海马组织中Caspase-3蛋白表达电泳图 Fig. 4 Electrophoregram of expressions of Caspase-3 protein in hippocampus tissue of rats in various groups |
|
|
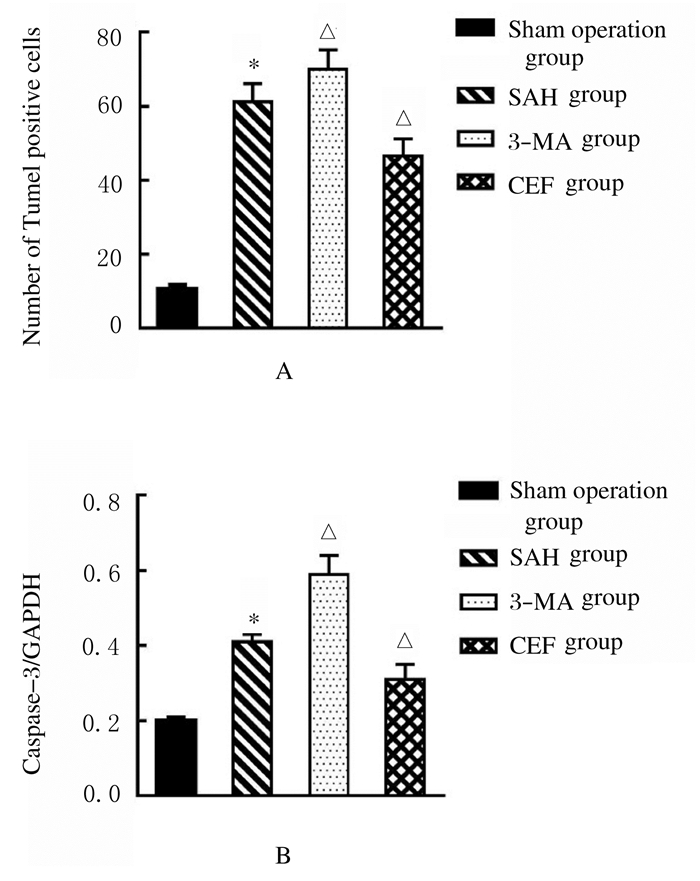
|
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with SAH group. 图 5 各组大鼠海马组织中凋亡细胞数(A)和Caspase-3蛋白表达水平(B) Fig. 5 Number of apoptotic cells and expression levels of Caspase-3 protein in hippocampus tissue of rats in various groups |
|
|
SAH是常见的脑血管疾病,在发病过程中会出现颅内压增高和脑疝,直接危及患者生命,而且大部分患者治愈后仍然会出现神经系统后遗症[9]。目前,国内外多数学者认为其可能与EBI有密切关联[10]。本研究中,HE染色观察到SAH组大鼠建模后24h脑组织出现明显的病理学改变,表明SAH可导致脑损伤。CEF是经美国食品药物监督管理局(FDA)批准的一种β-内酰胺类抗生素[11],已有研究[6, 12]报道:CEF可以升高谷氨酸转运蛋白(glutamate transporter-1, GLT-1)的表达,体内实验和体外实验均证实CEF有神经保护作用。KRZYZANOWSKA等[13]发现:CEF可以明显减轻脑缺血再灌注大鼠的神经功能损伤。本研究采用CEF对SAH大鼠治疗后,脑受损情况明显减轻,表现为脑组织病理改变减轻,凋亡细胞数量减少,表明CEF对SAH导致的EBI具有明显的保护作用。
自噬是胞质内大分子物质和细胞器在膜包囊泡中大量降解的生物学过程,普遍存在于真核生物的生命活动中[14]。自噬可以清除细胞中长寿命蛋白质和衰老的细胞器,以维持细胞基因组的稳定,促进细胞存活,是机体应激条件下的适应性反应[15]。目前诸多研究认为自噬是一把双刃剑,一定程度的自噬对细胞的存活起保护作用,也有研究认为自噬是一种细胞死亡方式,过度自噬可以加重组织损伤。LIU等[16]研究发现:大鼠脑缺血损伤后神经细胞自噬明显增强,抑制自噬脑缺血损伤进一步加重,提示脑缺血诱导的自噬与神经功能损伤有密切关联。LEE等[17]在SAH大鼠模型中发现:EBI损伤过程中自噬水平上调,损伤后24h达到峰值,使用自噬抑制剂3-MA干预后神经功能损伤明显加重。本研究结果显示:SAH后神经细胞自噬激活;CEF治疗后自噬标志性蛋白LC3-Ⅱ和Beclin-1蛋白表达水平进一步升高,HE染色示大鼠海马组织中坏死神经细胞数明显减少,提示CEF治疗可减少坏死神经细胞数,发挥保护作用,其可能与适度激活神经细胞自噬水平有关。
凋亡是细胞的一种程序性死亡方式[18],也是SAH的病理生理机制之一,神经细胞凋亡也是EBI损伤机制之一,抑制凋亡可减轻EBI直接造成的神经损伤[19]。SAH可以导致脑神经细胞缺血、缺氧,引发细胞内外环境的改变,启动凋亡相关通路,促进神经细胞死亡[20]。Caspase-3是凋亡通路中的关键因子,对凋亡的启动具有重要作用[21]。本研究结果显示:SAH组大鼠脑组织中凋亡细胞数明显升高,Caspase-3表达水平明显升高,经CEF治疗后凋亡细胞数明显减少,脑组织病理损伤减轻,提示CEF可能通过调节凋亡过程发挥神经保护作用。
生理状况下,机体细胞均存在一定水平的自噬和凋亡,以维持细胞内环境的稳态和自我更新及自身修复;当受到病理因素刺激时,自噬和凋亡水平均会提高,以应对病理性刺激,自噬和凋亡之间存在平衡,否则会加速细胞死亡[22]。自噬和凋亡已经在诸多神经系统疾病如脑卒中和脑外伤[23-24]中报道。本研究结果显示:SAH的EBI损伤可以诱导自噬和凋亡的增强,经CEF治疗的SAH大鼠神经细胞自噬水平增强,凋亡细胞数量减少。SAH为损伤性病理刺激,细胞内短时间聚集大量的受损细胞器及变性蛋白等,从而激活自噬和凋亡,但受损的细胞不足以维持自噬与凋亡之间的平衡,此过程加速了细胞的死亡。CEF治疗后可能稳定了细胞结构和功能,进一步促进自噬的激活,加速清除受损细胞器和变性蛋白,从而减少了细胞的凋亡。CEF使自噬循环流畅,起到神经保护作用。
综上所述,本研究仅对CEF干预下的自噬和凋亡进行了探讨,而未对其具体的调控机制进行深入研究,本课题组今后将深入探讨其调控机制,为CEF在SAH早期治疗中的应用奠定实验基础。
| [1] | YANG S, TANG WZ, HE Y C, et al. Long non-coding RNA and microRNA-675/let-7a mediates the protective effect of melatonin against early brain injury after subarachnoid hemorrhage via targeting TP53 and neural growth factor[J]. Cell Death Dis, 2018, 9(2): 99. DOI:10.1038/s41419-017-0155-8 |
| [2] | YANG S, CHEN X P, LI S L, et al. Melatonin treatment regulates SIRT3 expression in early brain injury (EBI) due to reactive oxygen species (ROS) in a mouse model of subarachnoid hemorrhage (SAH)[J]. Med Sci Monit, 2018, 24: 3804–3814. DOI:10.12659/MSM.907734 |
| [3] | LIU J X, ZHOU S, ZHANG Y T, et al. Bax inhibitor-1 suppresses early brain injury following experimental subarachnoid hemorrhage in rats[J]. Int J Mol Med, 2018, 42(5): 2891–2902. |
| [4] | 莫杭波.头孢曲松在蛛网膜下腔出血早期脑损伤中的保护作用及机制研究[D].杭州: 浙江大学, 2016. |
| [5] | FENG D, WANG W, DONG Y, et al. Ceftriaxone alleviates early brain injury after subarachnoid hemorrhage by increasing excittory amino acid transporter 2 expression via the PI3K/Akt/NF-κB signaling pathway[J]. Neuroscience, 2014, 268: 21–32. DOI:10.1016/j.neuroscience.2014.02.053 |
| [6] | KRZYZANOWSKA W, POMIERNY B, BYSTROWSKA B, et al. Ceftriaxone- and N-acetylcysteine-infduced brain tolerance to ischemia:Influence on glutamate levels in focal cerebral ischemia[J]. PLoS One, 2017, 12(10): e0186243. DOI:10.1371/journal.pone.0186243 |
| [7] | BARRY K J, GOGJIAN M A, STEIN B M. Small animal model for investigation of subarachnoid hemorrhage and cerebral vasospasm[J]. Stroke, 1979, 10(5): 538–541. DOI:10.1161/01.STR.10.5.538 |
| [8] | GARCIA J H, WAGNER S, LIU K F, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats[J]. Stroke, 1995, 26(4): 627–635. DOI:10.1161/01.STR.26.4.627 |
| [9] | AYAZ M, YANARDAG S B. SAH-induced electrophysiological changes of ventricular myocytes and role of N-acetylcysteine protection[J]. J Neurol Surg A Cent Eur Neurosurg, 2019, 80(2): 72–80. DOI:10.1055/s-0038-1655739 |
| [10] | NISHIKAWA H, LIU L, NAKANO F, et al. Modified citrus pectin prevents blood-brain barrier disruption in mouse subarachnoid hemorrhage by inhibiting galectin-3[J]. Stroke, 2018, 49(11): 2743–2751. DOI:10.1161/STROKEAHA.118.021757 |
| [11] | KUNDRA P, VAITHILINGAM B, VINAYAGAM S, et al. Ceftriaxone concentration at the surgical site following systemic and isolated upper limb injection[J]. J Anaesthesiol Clin Pharmacol, 2018, 34(3): 314–317. |
| [12] | ALSHEHRI F S, HAKAMI A Y, ALTHOBAITI Y S, et al. Effects of ceftriaxone on hydrocodone seeking behavior and glial glutamate transporters in P rats[J]. Behav Brain Res, 2018, 347: 368–376. DOI:10.1016/j.bbr.2018.03.043 |
| [13] | KRZYZANOWSKA W, POMIERNY B, BUDZISZEWSKA B, et al. N-acetylcysteine and ceftriaxone as preconditioning strategies in focal brain ischemia:influence on glutamate transporters expression[J]. Neurotox Res, 2016, 29(4): 539–550. DOI:10.1007/s12640-016-9602-z |
| [14] | MIZUSHIMA N, LEVINE B, CUERVO A M, et al. Autophagy fights disease through cellular self-digestion[J]. Nature, 2008, 451(7182): 1069–1075. DOI:10.1038/nature06639 |
| [15] | LIANG X H, JACKSON S, SEAMAN M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1[J]. Nature, 1999, 402(6762): 672–676. DOI:10.1038/45257 |
| [16] | LIU P F, LIU P J, WANG Z Y, et al. Inhibition of MicroRNA-96 ameliorates cognitive impairment and inactivation autophagy following chronic cerebral hypoperfusion in the rat[J]. Cell Physiol Biochem, 2018, 49(1): 78–86. |
| [17] | LEE J Y, HE Y D, SAGHER O, et al. Activated autophagy pathway in experimental subarachnoid hemorrhage[J]. Brain Res, 2009, 1287: 126–135. DOI:10.1016/j.brainres.2009.06.028 |
| [18] | 陈朝晖, 洪溪屏, 兰频, 等. 三肽脯氨酸对大鼠蛛网膜下腔出血后脑神经细胞凋亡的影响[J]. 浙江医学, 2018, 40(14): 1562–1566. DOI:10.12056/j.issn.1006-2785.2018.40.14.2017-1452 |
| [19] | MO J, ENKHJARGAL B, TRAVIS Z D, et al. AVE 0991 attenuates oxidative stress and neuronal apoptosis via Mas/PKA/CREB/UCP-2 pathway after subarachnoid hemorrhage in rats[J]. Redox Biol, 2019, 20: 75–86. DOI:10.1016/j.redox.2018.09.022 |
| [20] | YU T, FAN Y R, XU Y F, et al. Standardized Ginkgo biloba extract EGb 761 attenuates early brain injury following subarachnoid hemorrhage via suppressing neuronal apoptosis through the activation of Akt signaling[J]. Biomed Pharmacother, 2018, 107: 329–337. DOI:10.1016/j.biopha.2018.08.012 |
| [21] | CHEKENI F B, ELLIOTT M R, SANDILOS J K, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis[J]. Nature, 2010, 467(7317): 863–867. DOI:10.1038/nature09413 |
| [22] | CUGOLA F R, FERNANDES I R, RUSSO F B, et al. The Brazilian Zika virus strain causes birth defects in experimental models[J]. Nature, 2016, 534(7606): 267–271. DOI:10.1038/nature18296 |
| [23] | JIN Y C, WANG R, YANG S F, et al. Role of Microglia Autophagy in Microglia Activation After Traumatic Brain Injury[J]. World Neurosurg, 2017, 100: 351–360. DOI:10.1016/j.wneu.2017.01.033 |
| [24] | DUAN X C, WEN Z J, SHEN H T, et al. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy[J]. Oxid Med Cell Longev, 2016, 2016: 1203285. |
 2019, Vol. 45
2019, Vol. 45


