扩展功能
文章信息
- 赵丽艳, 宋扬, 陈勇, 贾茗博, 李蕴潜
- ZHAO Liyan, SONG Yang, CHEN Yong, JIA Mingbo, LI Yunqian
- 沉默ZEB1基因对胶质瘤U87细胞上皮-间质转化的影响
- Effect of silencing ZEB1 gene on epithelial to mesenchymal transition in glioma U87 cells
- 吉林大学学报(医学版), 2019, 45(05): 1036-1040
- Journal of Jilin University (Medicine Edition), 2019, 45(05): 1036-1040
- 10.13481/j.1671-587x.20190511
-
文章历史
- 收稿日期: 2019-01-18
2. 吉林大学第一医院神经外科, 吉林 长春 130021
2. Department of Neurosurgery, First Hospital, Jilin University, Changchun 130021, China
脑胶质瘤是最常见的原发性恶性肿瘤,具有高度侵袭性生长的病理特性,但其侵袭性生长的机制尚未阐明。上皮-间质转化(epithelial to mesenchymal transition,EMT)的肿瘤细胞呈高迁移性和高侵袭性,对肿瘤的侵袭、转移和复发具有重要作用[1]。本课题组前期工作和其他学者的研究[2-4]已表明:胶质瘤细胞能发生EMT,其可能在胶质瘤侵袭性生长中起重要作用。诱导EMT的转录因子(EMT-inducing transcription factors)通过转录调控EMT相关分子的表达,在诱导和激活EMT中起关键作用[5]。锌指E盒结合同源框(zinc finger E-box binding homeobox, ZEB)是重要诱导EMT转录因子之一,包括ZEB1和ZEB2。目前已有研究[6-7]证明:ZEB1能诱导肺癌、胰腺癌和乳腺癌细胞EMT,并促进肿瘤转移,但ZEB1对胶质瘤细胞EMT的影响尚未见研究报道。本研究使用短发卡RNA(shRNAs)沉默ZEB1基因表达,观察ZEB1对胶质瘤U87细胞EMT的影响,明确ZEB1在促进胶质瘤侵袭性生长中的可能作用。
1 材料与方法 1.1 细胞、主要试剂和仪器人恶性脑胶质瘤U87细胞(美国ATCC细胞库)。DMEM培养基、胎牛血清和胰酶-EDTA消化液(美国Gibco公司),青霉素和链霉素(美国Sigma公司),抗ZEB1抗体、抗N-钙黏蛋白、抗波形蛋白和抗基质金属蛋白酶9(matrix metalloproteinase-9, MMP-9)抗体(美国Cell Signaling Technology公司),抗β-tubulin抗体(美国Origene公司),TGF-β1(美国PeproTech公司),RIPA裂解液、BCA蛋白测定试剂盒、SDS和上样缓冲液(上海碧云天有限公司),蛋白Marker(美国Thermo公司),转染试剂TransLipid HL(北京全式金生物技术公司)。细胞培养箱和超净工作台(美国Thermo公司),倒置显微镜(日本Olympus公司),电泳仪和转膜仪(美国Bio-Rad公司),台式低速和高速离心机(美国Beckman公司)。
1.2 胶质瘤细胞培养U87细胞用含10%胎牛血清、1%青霉素和链霉素的DMEM培养基常规培养,每2~3 d更换培养基,每隔3 d按1:2~1:3传代,取对数生长期细胞进行后续实验。
1.3 ZEB1 shRNA干扰质粒的构建及转染ZEB1 shRNA干扰质粒由上海吉凯基因化学技术有限公司构建。取对数生长期的U87细胞接种于6孔板中,每孔为2×105个细胞,细胞融合约80%时进行质粒转染。按照TransLipid HL转染试剂说明书将ZEB1 shRNAs质粒(分别称shZEB1#1和shZEB1#2)和对照质粒shRNA(shCtrl)转染至U87细胞。质粒为4 μg,转染试剂体积为8~20 μL。转染4~6 h后,更换新的完全培养基,培养48 h后采用Western blotting法检测ZEB1蛋白表达水平,确定ZEB1 shRNAs转染效果。
1.4 Western blotting法鉴定ZEB1干扰效果和检测间质标志物的表达水平将U87细胞接种在10 cm培养皿(5×105个细胞/皿),37℃、5%CO2培养箱培养,待细胞融合达70%~80%时,换无血清培养基培养12 h,弃掉培养基,离心后收集细胞。加入RIPA裂解液提取蛋白质,采用BCA法测定蛋白质浓度后上样,SDS-PAGE电泳分离蛋白质,湿转至PVDF膜,5%脱脂奶粉室温封闭1 h,分别加入一抗ZEB1、N-钙黏蛋白、波形蛋白、MMP-9和β-tubulin,4℃过夜,洗膜,加入二抗37℃孵育,洗膜,加ECL显色剂,在凝胶成像系统中显影,检测各条带的灰度值,β-tubulin作为内参照。目的蛋白相对表达水平=目的条带灰度值/β-tubulin条带灰度值。
1.5 实验分组胶质瘤U87细胞分为对照组(胶质瘤U87细胞转染shCtrl)、EMT组(转染shCtrl的U87细胞用10×10-6g·L-1 TGF-β1处理48 h诱导EMT)和ZEB1基因沉默组(转染shZEB1的U87细胞用TGF-β1诱导EMT)。
1.6 细胞划痕法检测细胞迁移率取上述3组U87细胞接种于6孔培养板(每孔3×105个细胞),采用划痕试验检测和计算各组细胞的迁移率[8]。
1.7 统计学分析采用SPSS 19.0统计软件进行统计学分析。ZEB1、N-钙黏蛋白、波形蛋白和MMP-9蛋白表达水平及细胞迁移率以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用SNK-q检验。以P<0.05为差异有统计学意义。
2 结果 2.1 沉默ZEB1基因的胶质瘤细胞中ZEB1蛋白表达水平采用Western blotting法检测转染shCtrl和shZEB1的U87细胞中ZEB1蛋白表达水平,结果显示:2个短发卡RNA(shZEB1#1和shZEB1#2)均能抑制U87细胞中ZEB1蛋白表达水平,转染shZEB1#1和shZEB1#2 U87细胞中ZEB1蛋白表达水平(0.69±0.07和0.49±0.06)均较转染shCtrl的U87细胞(0.92±0.08)明显降低(P < 0.05和P < 0.01),表明ZEB1已稳定转染到U87细胞,成功建立了沉默ZEB1的胶质瘤U87细胞。与转染shZEB1#1的U87细胞(0.69±0.07)比较,转染shZEB1#2的U87细胞中ZEB1蛋白表达水平(0.49±0.06)明显降低(P < 0.05)。表明shZEB1#2对ZEB1表达的抑制效果更明显。在以下实验中使用shZEB1#2。见图 1。
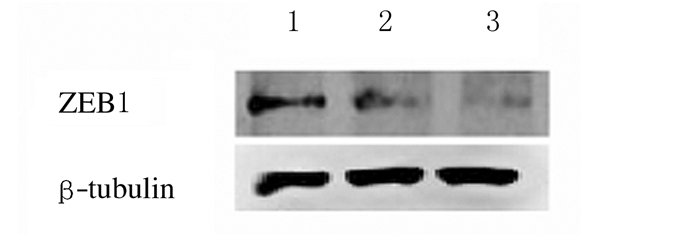
|
| 图 1 Western blotting法检测各组胶质瘤U87细胞中ZEB1蛋白表达电泳图 Fig. 1 Electrophoregram of expressions of ZEB1 protein in glioma cells in various groups detected by Western blotting method |
|
|
与对照组比较,EMT组U87细胞中N-钙黏蛋白、波形蛋白和MMP-9蛋白表达水平均明显升高(P < 0.05或P < 0.01);与EMT组比较,ZEB1基因沉默组细胞中N-钙黏蛋白、波形蛋白和MMP-9蛋白表达水平明显降低(P < 0.05或P < 0.01)。见图 2和表 1。

|
| 图 2 各组胶质瘤U87细胞中N-钙黏蛋白、波形蛋白和MMP-9蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of N-cadherin, Vimentin and MMP-9 proteins in glioma U87 cells in various groups |
|
|
| (n=3, x±s) | |||
| Group | N-cadherin | Vimentin | MMP-9 |
| Control | 0.97±0.13 | 0.98±0.16 | 0.97±0.07 |
| EMT | 1.37±0.14* | 1.51±0.10** | 1.24±0.09* |
| ZEB1 silence | 0.83±0.14△△ | 1.25±0.08△ | 0.73±0.10△△ |
| * P < 0.05, ** P < 0.01 vs control group; △P < 0.05, △△ P < 0.01 vs EMT group. | |||
EMT组细胞迁移率(69%±7%)明显高于对照组(51%±5%, P < 0.01),而ZEB1基因沉默组细胞迁移率(38%±4%)较EMT组明显降低(P < 0.01)。见图 3。

|
| A-C: 0 h; D-F: 24 h; A, D: Control group; B, E: EMT group; C, F: ZEB1 silence group 图 3 划痕24 h后各组胶质瘤U87细胞的迁移距离 Fig. 3 Migration distances of glioma U87 cells at 24 h after scratch in various groups |
|
|
本研究Western blotting法检测结果显示:转染shZEB1#1和shZEB1#2的U87细胞中ZEB1蛋白表达水平均明显降低,与shZEB1#1比较,shZEB1#2对ZEB1表达的抑制效果更为明显,表明ZEB1已稳定转染至U87细胞,成功建立了沉默ZEB1的胶质瘤U87细胞。
EMT是肿瘤细胞侵袭和转移的关键环节。恶性胶质瘤的一个显著特点是向周围组织侵袭性生长,近年来大量研究[9-10]表明:EMT这一病理生理过程在胶质瘤的侵袭性生长中起重要作用。有多种因素能诱导EMT过程,一些转录因子是EMT的重要诱导因子,如ZEB1、ZEB2、Snail、Slug和Twist1等,被称为诱导EMT转录因子或EMT激活剂(EMT-activator)。诱导EMT的转录因子能下调上皮标志蛋白的表达和(或)上调间质标志蛋白的表达,诱导EMT。通过抑制这些EMT诱导转录因子的表达及活性进而抑制并阻止EMT[5],已成为控制肿瘤细胞迁移和转移的策略之一。转录因子ZEB1在肿瘤发生发展中具有多方面作用,最主要的是促进肿瘤细胞EMT[11]。以往研究[7, 12]证明:在胰腺癌模型中使ZEB1失活可以消除侵袭和转移,抑制ZEB1活性可逆转肺癌细胞发生EMT。有研究[13-14]表明:ZEB1对恶性胶质瘤的起动、侵袭和耐药均发挥重要影响,用SHP-2上调ZEB1过表达能增强胶质瘤细胞侵袭和生长。最近对肝癌的研究[15]证明:ZEB1能与波形蛋白启动子中的某个位点结合,并调节波形蛋白基因的转录,这可能是沉默ZEB1基因表达能抑制EMT的分子机制之一。本研究结果显示:应用shZEB1抑制ZEB1基因表达,可明显降低U87细胞中间质标志物N-钙黏蛋白和波形蛋白的表达水平,表明沉默ZEB1能抑制胶质瘤细胞EMT。
基底膜降解是肿瘤侵袭的关键环节。MMP-9是明胶酶的一种,属于侵袭迁移相关蛋白,通过降解Ⅳ型胶原等基膜成分促进肿瘤的侵袭和转移[16]。有研究者[17-18]认为:MMP-9本身就是间质标志物,可以诱导EMT或EMT相关进程。本研究结果显示:应用shZEB1抑制ZEB1基因表达能明显降低胶质瘤细胞中MMP-9蛋白表达水平,提示抑制ZEB1能降低胶质瘤细胞的侵袭能力。增加细胞迁移能力是细胞发生EMT的重要表现之一。本研究结果表明:ZEB1沉默组的细胞迁移率较EMT组明显降低(P < 0.01),表明抑制ZEB1基因表达可以降低U87细胞的迁移能力。
综上所述,本研究结果证明沉默ZEB1基因表达能抑制胶质瘤U87细胞EMT,并降低细胞的迁移能力,提示转录因子ZEB1在促进胶质瘤侵袭性生长中扮演重要角色,可作为高度侵袭性胶质瘤治疗的重要靶标。
| [1] | IWATSUKI M, MIMORI K, YOKOBORI T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance[J]. Cancer Sci, 2010, 101(2): 293–299. DOI:10.1111/j.1349-7006.2009.01419.x |
| [2] | 宋扬, 赵丽艳, 陈勇, 等. TGFβ1对胶质瘤U87细胞上皮-间质转化的诱导作用[J]. 吉林大学学报:医学版, 2016, 42(6): 1087–1091. |
| [3] | KAHLERT U D, NIKKHAH G, MACIACZYK J. Epithelial-to-mesenchymal (-like) transition as a relevant molecular event in malignant gliomas[J]. Cancer Lett, 2013, 331(2): 131–138. DOI:10.1016/j.canlet.2012.12.010 |
| [4] | LEE J K, JOO K M, LEE J, et al. Targeting the epithelial to mesenchymal transition in glioblastoma:the emerging role of MET signaling[J]. Onco Targets Ther, 2014, 7: 1933–1944. |
| [5] | TANIA M, KHAN M A, FU JJ. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer[J]. Tumour Biol, 2014, 35(8): 7335–7342. DOI:10.1007/s13277-014-2163-y |
| [6] | LARSEN J E, NATHAN V, OSBORNE J K, et al. ZEB1 drives epithelial-to-mesenchymal transition in lung cancer[J]. J Clin Invest, 2016, 126(9): 3219–3235. DOI:10.1172/JCI76725 |
| [7] | KREBS A M, MITSCHKE J, LASIERRA LOSADA M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer[J]. Nat Cell Biol, 2017, 19(5): 518–529. DOI:10.1038/ncb3513 |
| [8] | 赵丽艳, 宋扬, 陈勇, 等. 白藜芦醇对胶质瘤U87细胞上皮-间质转化的抑制作用[J]. 吉林大学学报:医学版, 2018, 44(5): 943–948. |
| [9] | ISER I C, PEREIRA M B, LENZ G, et al. The epithelial-to-mesenchymal transition-like process in glioblastoma:An updated systematic review and in silico investigation[J]. Med Res Rev, 2017, 37(2): 271–313. DOI:10.1002/med.21408 |
| [10] | 宋扬, 赵丽艳, 李蕴潜, 等. 胶质瘤细胞上皮-间质转化及靶向抑制策略的研究进展[J]. 吉林大学学报:医学版, 2017, 43(5): 1064–1066. |
| [11] | CARAMEL J, LIGIER M, PUISIEUX A. Pleiotropic roles for ZEB1 in cancer[J]. Cancer Res, 2018, 78(1): 30–35. DOI:10.1158/0008-5472.CAN-17-2476 |
| [12] | REN J, CHEN YT, SONG HZ, et al. Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line[J]. J Cell Biochem, 2013, 114(6): 1395–1403. DOI:10.1002/jcb.24481 |
| [13] | SIEBZEHNRUBL F A, SILVER D J, TUGERTIMUR B, et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance[J]. EMBO Mol Med, 2013, 5(8): 1196–1212. DOI:10.1002/emmm.201302827 |
| [14] | ZHANG L, ZHANG W, LI Y, et al. SHP-2-upregulated ZEB1 is important for PDGFRα-driven glioma epithelial-mesenchymal transition and invasion in mice and humans[J]. Oncogene, 2016, 35(43): 5641–5652. DOI:10.1038/onc.2016.100 |
| [15] | QIN Y, YU JX, ZHANG M, et al. ZEB1 promotes tumorigenesis and metastasis in hepatocellular carcinoma by regulating the expression of vimentin[J]. Mol Med Rep, 2019, 19(3): 2297–2306. |
| [16] | BJÖRKLUND M, KOIVUNEN E. Gelatinase-mediated migration and invasion of cancer cells[J]. Biochim Biophys Acta, 2005, 1755(1): 37–69. |
| [17] | KAUFHOLD S, BONAVIDA B. Central role of Snail1 in the regulation of EMT and resistance in cancer:a target for therapeutic intervention[J]. J Exp Clin Cancer Res, 2014, 33: 62. DOI:10.1186/s13046-014-0062-0 |
| [18] | RADISKY E S, RADISKY D C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer[J]. J Mammary Gland Biol Neoplasia, 2010, 15(2): 201–212. DOI:10.1007/s10911-010-9177-x |
 2019, Vol. 45
2019, Vol. 45


