扩展功能
文章信息
- 谢婧, 李丽华
- XIE Jing, LI Lihua
- 脱水穿心莲内酯对四氯化碳诱导的肝纤维化模型小鼠肝细胞凋亡的抑制作用及其机制
- Inhibitory effect of dehydroandrographolide on hepatocyte apoptosis induced by carbon tetrachloride in hepatic fibrosis model mice and its mechanism
- 吉林大学学报(医学版), 2019, 45(05): 1009-1014
- Journal of Jilin University (Medicine Edition), 2019, 45(05): 1009-1014
- 10.13481/j.1671-587x.20190506
-
文章历史
- 收稿日期: 2018-11-02
肝纤维化是一个复杂的病理生理过程,其主要特征性表现为肝内结缔组织异常增生,沉积。长期肝纤维化可导致肝硬化,甚至肝癌[1],患者病死率居高不下,因此迫切需要预防和治疗肝纤维化的药物。近年来,肝纤维化的发病机制被广泛研究。其中肝星状细胞在肝纤维化的发生发展过程中起重要作用,肝脏损伤时受损的肝细胞可释放炎症因子激活静止的肝星状细胞,而活化的肝星状细胞可分泌细胞外基质,从而导致肝纤维化[2-4]。穿心莲为爵床类植物,具有广泛的药理作用,在临床上常用于治疗炎症、高血压、心血管和肝脏疾病[5-8],脱水穿心莲内酯(dehydroandrographolide,DA)是影响其治疗作用的主要活性成分之一,具有抗炎和抗氧化等作用[9]。目前,关于DA抗纤维化方面的研究较少,其抗纤维化机制尚不明确。本研究以四氯化碳(CCl4)诱导的慢性肝纤维化模型小鼠作为研究对象,探讨DA对肝纤维化小鼠肝细胞凋亡及肝星状细胞活化的影响,为其临床治疗提供新的理论依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器6~8周龄C57BL/6J雄性小鼠30只,体质量为18~22 g,购于北京维通利华实验动物技术有限公司,动物许可证号:SCXX(京)2016-0006。DA购自中国医药生物制品控制研究所,CCl4(上海试剂一厂),丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)、超氧化物歧化酶(SOD)和丙二醛(MDA)测定试剂盒(南京建成生物工程研究所),8-氧代鸟嘌呤DNA糖基化酶1(Ogg1)、半胱氨酸蛋白酶-3(Caspase-3)、Bcl-2相关X蛋白(Bax)、B淋巴细胞瘤-2基因(Bcl-2)、转化生长因子β1(TGF-β1)、α平滑肌肌动蛋白(α-SMA)和甘油醛-3-磷酸脱氢酶(GAPDH)一抗(英国Abcam公司),小鼠二步法检测试剂盒(北京中杉金桥公司),天狼猩红染色试剂盒(北京索莱宝公司),二抗(美国Proteintech公司)。ECL发光成像系统(1708195,美国Bio-Rad公司),OlympusBX-53型显微镜(日本Olympus公司)。
1.2 实验动物分组及模型制备实验前将30只小鼠随机分为对照组、模型组和治疗组,每组10只,动物管理符合动物保护条例。模型组和治疗组小鼠腹腔注射20% CCl4(2 mL·kg-1, 用橄榄油1:4稀释),每周3次,持续4周,对照组小鼠给予等量的橄榄油。治疗组在每次CCl4注射后给予DA(100 mg·kg-1)灌胃,同时对照组和模型组灌服等体积生理盐水。各组小鼠分别于末次给药24 h后经眼球取血,断髓,收集血清和肝脏标本。
1.3 HE染色检测小鼠肝组织病理形态表现小鼠肝组织经4%多聚甲醛固定、石蜡包埋、切片,采用HE法染色,光学显微镜下观察肝组织病理形态表现。
1.4 天狼猩红染色检测小鼠肝组织病理形态表现小鼠肝组织采用4%多聚甲醛固定48 h后,30%蔗糖脱水,OCT包埋后冰冻切片,切片厚度为8 μm。切片用PBS浸泡5 min后,滴加天狼猩红染色液室温1 h,流水冲洗,乙醇梯度脱水,二甲苯透明,树脂封片,光学显微镜下观察小鼠肝组织病理形态表现。
1.5 小鼠血清中ALT和AST活性及肝组织匀浆中SOD活性和MDA水平测定各组小鼠摘除眼球取血,4℃冰箱静置2 h后2 000 r·min-1离心15 min,取血清用于测定ALT和AST水平,单位为U·L-1;取冻存的相应各组小鼠肝脏称质量,按照肝脏质量和生理盐水以1:9比例、3 000 r·min-1离心10 min, 取上清液4℃保存,按照试剂盒说明书进行肝组织匀浆中SOD活性和MDA水平测定,单位为U·mg-1。
1.6 Western blotting法检测小鼠肝组织中Ogg1、Caspase-3、Bax和Bcl-2蛋白表达水平将肝组织加入预冷的裂解液,冰上剪碎后超声破碎1 min,静置30 min后4℃、12 000 r·min-1离心30 min,取上清,BCA蛋白定量,加入蛋白上样缓冲液并煮沸5 min。取30 μg组织蛋白行SDS-PAGE凝胶电泳并转印至PVDF膜;10%小牛血清白蛋白室温封闭2 h,一抗孵育过夜,TBST洗膜5 min×3次,二抗孵育2 h,TBST洗膜5 min ×3次,ECL显色液显色,暗室曝光。使用Image J进行灰度分析,目的蛋白(Ogg1、Caspase-3、Bax和Bcl-2蛋白)条带灰度值与内参照(GAPDH)条带灰度值比较,进行半定量分析。
1.7 免疫组织化学法检测肝组织中TGF-β1和α-SMA蛋白表达情况按照试剂盒说明书操作,使用二步法对肝组织中TGF-β1和α-SMA蛋白进行染色,光学显微镜下观察到棕黄色或棕褐色颗粒者为TGF-β1和α-SMA蛋白阳性表达。
1.8 统计学分析采用SPSS 19.0统计软件进行统计学分析。血清中ALT和AST活性、肝组织中SOD活性和MDA水平以及肝组织中蛋白表达水平进行正态分布和方差齐性检验,均为正态分布及方差齐,以x±s表示,多组间样本均数比较采用单因素方差分析。以α=0.05为检验标准。
2 结果 2.1 各组小鼠血清中ALT和AST活性与对照组比较,模型组和治疗组小鼠血清中ALT和AST活性均明显升高(P < 0.05),表明肝细胞损伤增加;与模型组比较,治疗组ALT和AST活性明显降低(P < 0.05)。见表 1。
| [n=6, x ±s, λB/(U·L-1)] | ||
| Group | ALT | AST |
| Control | 40.95±5.43 | 22.34±2.35 |
| Model | 247.94±35.25* | 158.43±12.67* |
| Treatment | 131.65±8.64*△ | 51.82±6.85*△ |
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | ||
HE染色和天狼猩红染色结果显示:对照组小鼠肝组织结构正常,胶原纤维呈红色且仅存在于汇管区和中央静脉;与对照组比较,模型组小鼠肝脏组织肝小叶结构异常,肝细胞排列紊乱,大量炎性细胞浸润,汇管区可见纤维结缔组织增生,出现条索样结构,表明造模成功;与模型组比较,治疗组肝组织炎性细胞浸润和胶原沉积程度明显减轻。见图 1(插页一)。

|
| A-C:HE staining; D-F: Sirius red staining; A,D: Control group; B,E: Model group; C,F: Treatment group. 图 1 各组小鼠肝组织病理形态表现(×100) Fig. 1 Pathomorphology of liver tissue of mice in various groups(×100) |
|
|
与对照组比较,模型组小鼠肝组织中SOD活性降低(P < 0.05),MDA水平明显升高(P < 0.05);与模型组比较,治疗组小鼠肝组织中SOD活性明显升高(P < 0.05),MDA水平明显降低(P < 0.05)。见表 2。
| [n=6, x ±s, λB/(U·mg-1)] | ||
| Group | SOD | MDA |
| Control | 22.97±2.31 | 17.31±1.67 |
| Model | 15.79±0.78* | 26.19±2.13* |
| Treatment | 19.12±0.53△ | 22.28±1.29△ |
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | ||
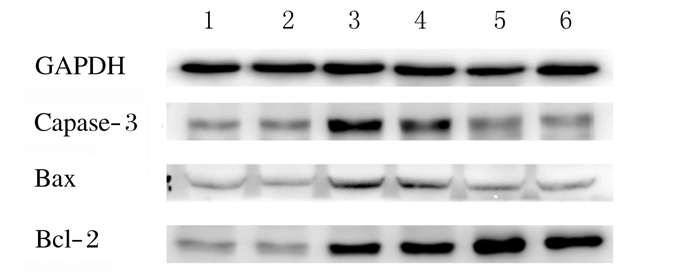
Western blotting法检测结果显示:与对照组(0.079±0.030)比较,模型组小鼠肝组织中Ogg1蛋白表达水平(0.256±0.050)明显升高(P < 0.05);治疗组小鼠肝组织中Ogg1蛋白表达水平(0.127±0.02)明显降低(P < 0.05)。见图 2。与对照组比较,模型组小鼠肝组织中Caspase-3、Bax和Bcl-2蛋白表达水平明显升高(P < 0.05);与模型组比较,治疗组小鼠肝组织中Caspase-3和Bax蛋白表达水平明显降低(P < 0.05),Bcl-2蛋白表达水平明显升高(P < 0.05)。见图 3和表 3。

|
| Lane 1, 2: Control group; Lane 3, 4: Model group; Lane 5, 6: Treatment group. 图 2 各组小鼠肝组织中Ogg1蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of Ogg1 proteinin liver tissue of mice in various groups |
|
|
|
| Lane 1, 2: Control group; Lane 3, 4: Model group; Lane 5, 6: Treatment group. 图 3 各组小鼠肝组织中Caspase-3、Bax和Bcl-2蛋白表达电泳图 Fig. 3 Electrophoregram of expressions of Caspase-3, Bax, and Bcl-2 proteins in liver tissue of mice in various groups |
|
|
| (n=6, x ±s) | |||
| Group | Caspase-3 | Bax | Bcl-2 |
| Control | 1.038±0.020 | 0.652±0.020 | 1.052±0.040 |
| Model | 1.751±0.030* | 1.061±0.020* | 1.701±0.030* |
| Treatment | 1.118±0.010△ | 0.678±0.020△ | 2.083±0.030△ |
| * P < 0.05 compared with control group; △ P < 0.05 compared with model group. | |||
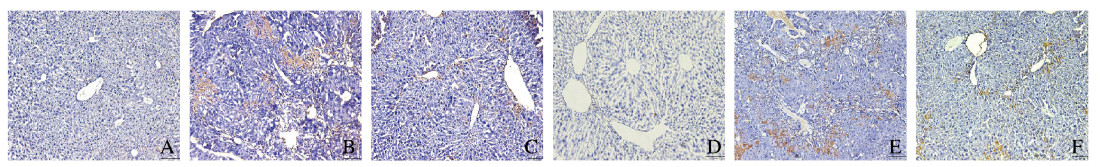
免疫组织化学法检测结果显示:胞浆或胞核呈棕褐色染色,表明小鼠肝组织中TGF-β1和α-SMA蛋白阳性表达。对照组小鼠肝组织中TGF-β1和α-SMA蛋白阳性表达量较低;模型组小鼠肝组织中TGF-β1和α-SMA蛋白阳性表达量明显增加;与模型组小鼠比较,治疗组小鼠肝组织TGF-β1和α-SMA蛋白阳性表达量明显降低。见图 4(插页二)。
|
| A-C: TGF-β1; D-F: α-SMA; A, D: Control group; B, E: Model group; C, F: Treatment group. 图 4 各组小鼠肝组织中TGF-β1和α-SMA蛋白的表达(免疫组织化学,×100) Fig. 4 Expressions of TGF-β1 and α-SMA proteins in liver tissue of mice in various groups(Immunohistochemistry, ×100) |
|
|
肝纤维化是各种慢性肝脏疾病发展为肝硬化的关键环节,其本质是肝组织内细胞外基质过度增生和沉积,导致纤维结缔组织增生[10]。肝纤维化过程中伴随着肝细胞损伤、氧化应激以及肝星状细胞活化[11]。目前穿心莲在临床上常用于治疗肝脏疾病,其主要活性成分为穿心莲内酯和DA。关于穿心莲的研究主要集中在穿心莲内酯方面,研究[12-14]表明:穿心莲内酯能够通过降低炎症介质的表达,减轻CCl4、刀豆球蛋白A和α-异硫氰酸酯引起的肝损伤,达到抗炎护肝的作用。为此本文作者推测穿心莲的另一活性成分DA在慢性肝纤维化模型中是否也可起到护肝作用。前期实验已证明DA在100 mg·kg-1时对小鼠慢性肝脏损伤具有明显的抗炎作用[9]。本研究结果显示:DA治疗后小鼠血清中ALT和AST活性明显降低;组织病理学观察显示:DA可以明显减轻小鼠肝组织炎性细胞浸润和胶原沉积,表明DA能够有效地减轻CCl4诱导的肝损伤及肝纤维化,对CCl4引起的小鼠慢性肝纤维化具有治疗作用。
CCl4诱导肝纤维化是较为经典的建立肝纤维化动物模型的方法。CCl4经腹腔注射进入动物体内后,在肝细胞内被细胞色素P450氧化酶激活后,产生CCl3,而CCl3可引起脂质过氧化和活性氧自由基的产生,从而损伤肝细胞[15-17]。肝细胞损伤是导致肝纤维化的起始因素,又是推动其发展的必要因素[18]。肝细胞损伤尤其是肝细胞凋亡具有促纤维化的作用,因此减少肝细胞凋亡可减轻肝纤维化。细胞凋亡由多种基因参与调控,如Bcl-2家族、Caspase家族和抑癌基因p53。在细胞凋亡中死亡受体途径与线粒体途径均能激活Caspase-3, 诱导细胞凋亡。Bcl-2家族在调控细胞凋亡过程中也发挥着重要作用[19],Bcl-2家族中Bax为促凋亡因子、Bcl-2为抑凋亡因子,而Bax可拮抗Bcl-2抑制细胞凋亡的作用,Bax与Bcl-2蛋白结合形成异二聚体,使Bcl-2失活,从而加速细胞凋亡。在细胞凋亡发展中,Bax和Bcl-2表达水平各不相同,而Bax和Bcl-2蛋白表达水平的结果决定了细胞的存活或凋亡[20-22]。本研究结果显示:与对照组比较,模型组小鼠血清中SOD活性降低,MDA和Ogg1水平升高;与模型组比较,治疗组小鼠血清中SOD活性升高,MDA和Ogg1表达水平降低,表明DA对CCl4诱导的慢性肝损伤小鼠的保护作用可能是通过抑制CCl4导致的氧化应激损伤完成的;与模型组比较,治疗组小鼠肝组织中Bax表达水平降低,Bcl-2表达水平升高,同时Caspase-3表达水平也明显降低,提示DA治疗后肝组织中Bcl-2/Bax比值上调,降低了异二聚体形成,从而减少肝细胞的凋亡。
肝细胞在受到化学刺激或损伤时,会导致细胞发生凋亡,而凋亡的肝细胞可产生促纤维因子(TGF-β1)[2]。在正常状态下肝星状细胞处于静息状态,TGF-β1可作用于肝星状细胞,使其转化为活化状态。给予DA治疗后能明显降低TGF-β1蛋白表达水平。肝细胞损伤可诱导细胞因子或生长因子释放,从而诱导肝星状细胞活化,导致α-SMA蛋白表达水平升高,细胞外基质沉积,因此α-SMA蛋白可以被认为是肝星状细胞活化的标志。本研究结果显示:与模型组比较,治疗组小鼠肝组织中TGF-β1和α-SMA阳性表达量明显降低,提示DA可能是通过减少TGF-β1生成,抑制肝星状细胞活化,从而减轻CCl4导致的肝纤维化。
综上所述,DA可以通过下调氧化应激蛋白表达,降低肝细胞凋亡,进而减少肝星状细胞活化,从而发挥抗纤维化作用。
| [1] | WANG R, YU X Y, GUO Z Y, et al. Inhibitory effects of salvianolic acid B on CCl(4)-induced hepatic fibrosis through regulating NF-κB/IκBα signaling[J]. J Ethnopharmacol, 2012, 144(3): 592–598. DOI:10.1016/j.jep.2012.09.048 |
| [2] | TANG L Y, HELLER M, MENG ZJ, et al. Transforming growth factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway[J]. J Biol Chem, 2017, 292(10): 4302–4312. DOI:10.1074/jbc.M116.773085 |
| [3] | CHEN YH, WU ZF, YUAN BY, et al. MicroRNA-146a-5p attenuates irradiation-induced and LPS-induced hepatic stellate cell activation and hepatocyte apoptosis through inhibition of TLR4 pathway[J]. Cell Death Dis, 2018, 9(2): 22. DOI:10.1038/s41419-017-0038-z |
| [4] | AL-RASHEED N, FADDAH L, AL-RASHEED N, et al. Protective effects of silymarin, alone or in combination with chlorogenic acid and/or melatonin, against carbon tetrachloride-induced hepatotoxicity[J]. Pharmacogn Mag, 2016, 12(Suppl 3): S337–S345. |
| [5] | TAO L, ZHANG L, GAO R, et al. Andrographolide alleviates acute brain injury in a rat model of traumatic brain injury:possible involvement of inflammatory signaling[J]. Front Neurosci, 2018, 12: 657. DOI:10.3389/fnins.2018.00657 |
| [6] | LIANG ES, LIU X, DU ZH, et al. Andrographolide ameliorates diabetic cardiomyopathy in mice by blockage of oxidative damage and NF-κB-mediated inflammation[J]. Oxid Med Cell Longev, 2018, 2018: 9086747. |
| [7] | HSIEH Y L, SHIBU M A, LⅡ C K, et al. Andrographis paniculata extract attenuates pathological cardiac hypertrophy and apoptosis in high-fat diet fed mice[J]. J Ethnopharmacol, 2016, 192: 170–177. DOI:10.1016/j.jep.2016.07.018 |
| [8] | CHEN Y Y, HSU M J, HSIEH C Y, et al. Andrographolide inhibits nuclear factor-κB activation through JNK-Akt-p65 signaling cascade in tumor necrosis factor-α-stimulated vascular smooth muscle cells[J]. Sci World J, 2014, 2014: 130381. |
| [9] | WENG ZY, CHI Y, XIE J, et al. Anti-inflammatory activity of dehydroandrographolide by TLR4/NF-κB signaling pathway inhibition in bile duct-ligated mice[J]. Cell Physiol Biochem, 2018, 49(3): 1083–1096. DOI:10.1159/000493292 |
| [10] | SEO H Y, JUNG Y A, LEE S H, et al. Kahweol decreases hepatic fibrosis by inhibiting the expression of connective tissue growth factor via the transforming growth factor-beta signaling pathway[J]. Oncotarget, 2017, 8(50): 87086–87094. |
| [11] | DAS N, MANDALA A, NAAZ S, et al. Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice[J]. J Pineal Res, 2017, 62(4). DOI:10.1111/jpi.12404 |
| [12] | CHEN S R, LI F, DING M Y, et al. Andrographolide derivative as STAT3 inhibitor that protects acute liver damage in mice[J]. Bioorg Med Chem, 2018, 26(18): 5053–5061. DOI:10.1016/j.bmc.2018.09.002 |
| [13] | LEE T Y, LEE K C, CHANG H H. Modulation of the cannabinoid receptors by andrographolide attenuates hepatic apoptosis following bile duct ligation in rats with fibrosis[J]. Apoptosis, 2010, 15(8): 904–914. DOI:10.1007/s10495-010-0502-z |
| [14] | SHI G J, ZHANG Z J, ZHANG R, et al. Protective effect of andrographolide against concanavalin A-induced liver injury[J]. Naunyn Schmiedebergs Arch Pharmacol, 2012, 385(1): 69–79. DOI:10.1007/s00210-011-0685-z |
| [15] | SHAH B, SHAH G. Antifibrotic effect of heparin on liver fibrosis model in rats[J]. World J Gastrointest Pharmacol Ther, 2012, 3(6): 86–92. DOI:10.4292/wjgpt.v3.i6.86 |
| [16] | LI X X, ZHENG Q C, WANG Y, et al. Theoretical insights into the reductive metabolism of CCl4 by cytochrome P450 enzymes and the CCl4-dependent suicidal inactivation of P450[J]. Dalton Trans, 2014, 43(39): 14833–14840. DOI:10.1039/C4DT02065K |
| [17] | XIE Y, HAO H P, WANG H, et al. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress[J]. J Ethnopharmacol, 2014, 155(1): 213–221. DOI:10.1016/j.jep.2014.05.016 |
| [18] | TABET E, GENET V, TIAHO F, et al. Chlordecone potentiates hepatic fibrosis in chronic liver injury induced by carbon tetrachloride in mice[J]. Toxicol Lett, 2016, 255: 1–10. DOI:10.1016/j.toxlet.2016.02.005 |
| [19] | WU H, QIU Y, SHU Z Y, et al. Protective effect of Trillium tschonoskii saponin on CCl4-induced acute liver injury of rats through apoptosis inhibition[J]. Can J Physiol Pharmacol, 2016, 94(12): 1291–1297. DOI:10.1139/cjpp-2016-0228 |
| [20] | 陆璐, 武超, 谢咚, 等. 八宝丹对急性肝衰竭大鼠肝性脑病的防治作用[J]. 临床肝胆病杂志, 2018, 34(12): 2635–2641. DOI:10.3969/j.issn.1001-5256.2018.12.025 |
| [21] | WANG Y, WANG R, WANG Y, et al. Ginkgo biloba extract mitigates liver fibrosis and apoptosis by regulating p38 MAPK, NF-κB/IκBα, and Bcl-2/Bax signaling[J]. Drug Des Devel Ther, 2015, 9: 6303–6317. |
| [22] | MARIN J J, HERNANDEZ A, REVUELTA I E, et al. Mitochondrial genome depletion in human liver cells abolishes bile acid-induced apoptosis:role of the Akt/mTOR survival pathway and Bcl-2 family proteins[J]. Free Radic Biol Med, 2013, 61: 218–228. DOI:10.1016/j.freeradbiomed.2013.04.002 |
 2019, Vol. 45
2019, Vol. 45


