扩展功能
文章信息
- 刘晓华, 韩曼, 张琪
- LIU Xiaohua, HAN Man, ZHANG Qi
- 大鼠心脏TRPV1受体激活对心血管活动的抑制作用
- Inhibitory effect of activation of cardiac TRPV1 receptors on cardiovascular activity in rats
- 吉林大学学报(医学版), 2019, 45(05): 992-996
- Journal of Jilin University (Medicine Edition), 2019, 45(05): 992-996
- 10.13481/j.1671-587x.20190503
-
文章历史
- 收稿日期: 2019-02-23
心肌缺血时释放多种致痛物质激活分布于心脏感觉神经末梢的伤害性感受受体引发心绞痛[1]。心脏感觉传入纤维伴随心交感和心迷走神经传入中枢神经系统[2],除引起伤害性感觉之外,心脏伤害性刺激常诱发心血管反射[3]。研究[4-6]显示:刺激大鼠心脏特定区域可使心交感神经和心迷走神经紧张性活动发生改变,反射性地引起心率和血压变化。上述改变不论在生理状态下还是在病理状态下均具有重要的意义[7]。瞬时受体电位香草酸受体1(transient receptor potential vanilloid 1 receptor, TRPV1)是一种非选择性阳离子通道,可被辣椒素、伤害性热刺激和质子等激活[8]。TRPV1主要分布于感觉神经元及其纤维末梢,是机体重要的伤害性感受受体,参与多种病理生理过程[9-10]。研究[4]显示:大鼠心脏表面分布有大量TRPV1阳性感觉传入纤维。本课题组前期研究[6]显示:心包内注射辣椒素除引起心脏-躯体伤害性反射运动之外,同时还伴有心血管反射,但其具体机制尚不清楚。本研究通过大鼠心包内注射辣椒素激活心脏表面TRPV1受体,观察TRPV1受体拮抗剂和M受体拮抗剂对辣椒素诱发的心血管反射的影响以及延髓心血管中枢c-Fos蛋白阳性神经元与胆碱酯酶的共表达,以明确TRPV1受体激活诱发的心血管反射的神经机制。
1 材料与方法 1.1 实验动物、主要试剂和仪器选择健康、雄性SD大鼠60只,体质量260~330 g,购自成都达硕实验动物有限公司,动物合格证号:SCXK(川)2015-030。辣椒素和TRPV1受体拮抗剂——树胶脂毒素(ido-RTX)购自美国Sigma公司,兔抗鼠c-Fos一抗(AB7963)、羊抗鼠胆碱酯酶(choline acetyltransferase, ChAT)一抗(AB18736)、荧光二抗驴抗兔IgG(Alexa Fluor488)和驴抗羊IgG(Alexa Fluor 594)均购自美国Abcam公司,碳花青荧光染料(DiI)购自美国Thermo Fisher公司。BL-420生物信号采集与分析系统(成都泰盟生物科技有限公司),压力换能器(成都泰盟生物科技有限公司),BT100-2J蠕动泵(保定兰格恒流泵有限公司),DW3000-B型小动物呼吸机(淮北正华生物仪器设备有限公司),Kryostat 1720冰冻切片机(德国Leitz公司),Olympus生物显微镜BX51(日本Olympus公司)。辣椒素溶于吐温80和无水乙醇(按1:1的比例混合)溶液中,配制成1×10-3mg·L-1的贮存液;生理盐水新鲜配制浓度为1 mg·L-1;ido-RTX溶于DMSO,最终浓度为10 mg·L-1。
1.2 实验动物分组及心包插管术将60只实验大鼠随机分为对照组、ido-RTX组、阿托品组、免疫荧光组和神经束路追踪组,每组12只。各组大鼠均行心包插管手术。实验动物称质量后以初始剂量的戊巴比妥钠(45~55 mg·kg-1)腹腔麻醉后取仰卧位,于左侧上胸部第1~3肋软骨间行开胸术,暴露胸腺。中线处分离胸腺,暴露心包膜。玻璃分针尖端(直径0.5 mm)在心包膜上扎开一个小孔,将一长12~14 cm远端有数个小洞的硅胶管(内径0.020 cm、外径0.037 cm)经此孔插入心包约2 cm,缝合胸腺及各层胸壁组织以固定心包插管。ido-RTX组和阿托品组大鼠心包插管,行气管插管术后连接小动物呼吸机,动脉插管连接动脉血压换能器监测动脉血压,颈静脉插管维持麻醉(10~15 mg·kg-1·h-1)。手术后1 h待大鼠状态稳定后开始注射,同时记录动脉血压及心率的变化。注射顺序为溶酶、辣椒素、受体拮抗剂加辣椒素和辣椒素。受体拮抗剂于0.2 mL(1mg·L-1)辣椒素注射前10~15 min注射。辣椒素注射1 min后回抽并用生理盐水冲洗心包4~6次,间隔时间1 h。
1.3 免疫荧光染色检测延髓心血管中枢阳性细胞表达对照组及免疫荧光组大鼠行心包插管手术后,对照组大鼠心包内注射生理盐水0.2 mL,免疫荧光组大鼠心包内注射辣椒素0.2 mL,1 h后4%多聚甲醛灌注固定。取延髓置于4%多聚甲醛固定12 h后转移至30%蔗糖的沉底。恒冷箱连续切片(切片厚度25 μm),c-Fos一抗(1:1 000)与ChAT一抗(1:200)4℃共同孵育48 h。荧光二抗驴抗兔IgG与驴抗羊IgG室温下共孵育40 min后,PBST漂洗,裱片。荧光纤维镜下观察c-Fos阳性细胞和ChAT阳性神经元,拍照。神经束路追踪组大鼠行心包置管术后心包内注射荧光活性染料DiI (0.1 mL, 2.5 g·L-1)作为神经束路追踪剂,对照组大鼠心包内注射生理盐水。1周后4%多聚甲醛灌注固定后取延髓,恒冷箱切片(切片厚度25 μm),荧光显微镜下观察红色荧光标记的神经元并拍照。
1.4 统计学分析采用SPSS 20.0统计软件进行统计学分析。Image软件计数免疫荧光以及神经束路追踪阳性细胞。各组大鼠平均动脉压和心率,c-Fos阳性神经元、ChAT阳性神经元和Dil标记神经元数量以x±s表示,给药前后比较采用单因素重复测量方差分析,组间样本均数比较采用两独立样本t检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 ido-RTX组大鼠平均动脉压和心率辣椒素单独注射后ido-RTX组大鼠心率从[(363±15)min-1]下降到[(254±27)min-1],平均动脉压从[(101±3)mmHg]下降到[(66±3)mmHg] (P < 0.05)。ido-RTX组大鼠心包内预先注射ido-RTX再注射辣椒素,心率为[(358± 15)min-1],平均动脉压为[(98±3)mmHg],与注射前[心率(371±15)min-1和平均动脉压(106±3)mmHg]比较差异无统计学意义(P>0.05)。见图 1。
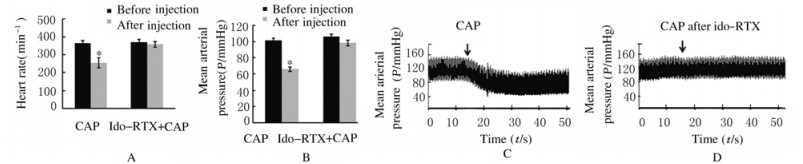
|
| A: Heart rate; B: Mean arterial pressure; C, D: Examples of blood pressure recordings. CAP:Capsaicin. *P < 0.05 vs before injection. 图 1 ido-RTX组大鼠心率和平均动脉压 Fig. 1 Heart rates and mean arterial pressures of rats in ido-RTX group |
|
|
阿托品组大鼠单独注射辣椒素时心率和平均动脉血压均较注射前降低(P < 0.05)。注射阿托品后再注射辣椒素,大鼠心率[(342±20)min-1]和平均动脉血压[(99±2)mmHg]与注射前比较差异无统计学意义(n=8,P>0.05)。见图 2。
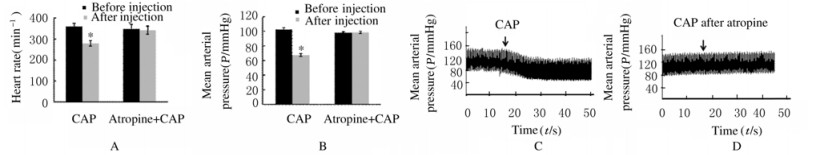
|
| A: Heart rate; B: Mean arterial pressure; C, D: Examples of recordings. CAP: Capsaicin. *P < 0.05 vs before injection. 图 2 阿托品组大鼠心率和平均动脉压 Fig. 2 Heart rates and mean arterial pressures of rats in atropine group |
|
|
心包内注射辣椒素后延髓心血管中枢c-Fos阳性细胞数较对照组明显升高(P < 0.01),主要表达于延髓孤束核、迷走神经背核和疑核,且大部分c-Fos阳性神经元为ChAT阳性神经元。见表 1和图 3(插页一)。

|
| A: ChAT; B: C-Fos; C:Brain map; D: Merged. The white arrows indicated the positive neurons. 图 3 各组大鼠迷走神经背核中C-Fos和ChAT的共表达(Bar = 50 μm) Fig. 3 Co-expression of c-Fos and ChAT in dorsal motor nucleus of vagus nerve of rats in groups (Bar = 50 μm) |
|
|
| (n=12, x ±s) | |||
| Area | NTS | DMV | NA |
| Control | 9±4 | 5±1 | 1±1 |
| Capsaicin | 142±10* | 56±8* | 14±3* |
| NTS: Nucleus tractus solitarii; DMV: Dorsal motor nucleus of vagus; NA: Nucleus ambiguous. * P < 0.05 vs control group. | |||
DiI荧光标记阳性神经元的分布心包内注射荧光染料DiI逆行束路追踪心包神经纤维的中枢投射部位,1周后荧光显微镜下观察显示:DiI阳性细胞主要出现在延髓孤束核、迷走神经背核和疑核神经元的胞体以及神经纤维末梢。见图 4(插页一)。
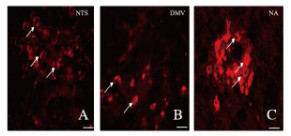
|
| A: NTS; B:DMV; C:NA.The white arrows indicated the positive neurons. 图 4 神经束路追踪组大鼠延髓心血管中枢DiI阳性神经元的分布(Bar=50 μm) Fig. 4 Distribution of DiI positive neurons in medulla oblongata cardiovascular center of rats in nerve tracing group(Bar=50 μm) |
|
|
本课题组前期研究[6]表明:心包内注射辣椒素可以激活心脏伤害性感受受体,诱发心脏-躯体运动反射,同时伴随心血管反射。本研究结果显示:心包内注射辣椒素诱发的心血管反射被TRPV1受体拮抗剂ido-RTX和M受体拮抗剂完全阻断,同时辣椒素诱发c-Fos蛋白在延髓心血管中枢表达;且与ChAT共表达;心包内注射神经束路追踪剂DiI后,延髓孤束核、疑核和迷走神经背核等核团出现DiI阳性细胞。上述结果提示:激活心脏TRPV1受体可使延髓心血管中枢胆碱能神经元的活动增强,外周释放乙酰胆碱增加抑制心血管活动。
TRPV1是一种重要的伤害性感受受体,约50%无髓传入神经纤维为TRPV1阳性神经纤维[11]。除参与伤害性感受信息的传递之外,越来越多的研究[7]表明:TRPV1参与多种生理病理过程。本研究中大鼠心包内注射辣椒素诱发的心血管反射被TRPV1特异性拮抗剂ido-RTX阻断,提示心脏TRPV1参与心血管活动的调节。延髓孤束核是心迷走神经传入纤维的主要投射部位,激活孤束核内TRPV1受体对心血管活动产生抑制作用,心率和平均动脉压均下降[12],提示外周和中枢的TRPV1均参与心血管活动的调节。
心血管活动主要受交感神经和迷走神经调节。交感神经兴奋其末梢释放去甲肾上腺素作用于肾上腺素能受体,对心血管产生明显的兴奋作用;迷走神经兴奋其末梢释放乙酰胆碱作用于M受体,对心血管活动产生抑制作用。正常生理状态下,交感神经和迷走神经相互制约,共同参与心血管活动的调节[13]。冠心病恶性猝死性心律失常以及心肌缺血等疾病发生时,交感神经的兴奋性增加,自主神经系统的稳态被破坏[14],此时维持迷走神经的兴奋性对自主神经系统功能活动意义重大。本研究中M受体拮抗剂阿托品阻断了TRPV1激活对心血管活动产生的抑制作用,表明激活心脏TRPV1可使心迷走神经的兴奋性增高,提示TRPV1传入纤维在迷走神经的兴奋性维持中扮演重要角色。近年来TRPV1受体的心肌保护作用越来越受到重视。TRPV1受体激活可使感觉神经末梢释放钙基因相关肽和P物质增加,对心肌缺血再灌注损伤产生明显的保护作用[15]。TRPV1介导的心迷走神经活动也可能是其产生心肌保护作用的机制之一[16]。
心包内注射作为一种安全、可重复性的操作方法,在心脏疾病的研究中被广泛应用[17]。既往对于心包的中枢神经纤维相关研究较少,本研究中大鼠心包内注射荧光活性染料DiI,观察延髓DiI阳性细胞在延髓心血管中枢的表达以明确心包在延髓的神经投射中枢,结果表明:DiI阳性细胞以及神经纤维末梢主要出现在延髓孤束核、迷走神经背核和疑核。孤束核是心脏感觉传入接替核,迷走神经背核以及疑核是心迷走神经的中枢,提示心包的中枢神经投射部位与心脏类似,心包内注射可以作为心脏疾病研究的有效手段。
本研究利用c-Fos蛋白作为神经元活动的化学标志,研究心包内注射辣椒素后延髓心血管中枢神经元的反应。心包内注射辣椒素后在延髓孤束核、迷走神经背核和疑核c-Fos阳性细胞数增多,提示上述核团参与TRPV1激活诱发的心血管反射。孤束核是内脏感觉在中枢的首要接替部位,在心脏伤害性信息的传递中扮演重要的角色[18-21]。心迷走神经直接投射到孤束核,心交感神经的传入纤维首先投射到脊髓,由脊髓中转后再投射到孤束核[2]。孤束核将外周信息整合中转到更高级中枢以及延髓心血管中枢的核团。疑核和迷走神经背核为心迷走神经的中枢,是产生和维持心迷走神经紧张性活动的重要部位[2]。TRPV1激活后疑核和迷走神经背核内c-Fos阳性细胞数上升且与胆碱酯酶ChAT共表达,表明心脏TRPV1传入兴奋可激活心迷走神经节前胆碱能神经元。对于TRPV1受体介导的心血管反射活动是否与心交感神经活动的抑制作用有关联,还需进一步的实验证明。
| [1] | FOREMAN RD, GARRETT KM, BLAIR RW. Mechanisms of cardiac pain[J]. Compr Physiol, 2015, 5(2): 929–960. |
| [2] | GIBBINS I. Functional organization of autonomic neural pathways[J]. Organogenesis, 2013, 9(3): 169–175. DOI:10.4161/org.25126 |
| [3] | TOSCHI-DIASE, RONDON M U P B, COGLIATI C, et al. Contribution of autonomic reflexes to the hyperadrenergic state in heart failure[J]. Front Neurosci, 2017, 11: 162. |
| [4] | ZAHNER M R, LI D P, CHEN S R, et al. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats[J]. J Physiol(Lond), 2003, 551(Pt2): 515–523. |
| [5] | CINCA J, RODRÍGUEZ-SINOVAS A. Cardiovascular reflex responses induced by epicardial chemoreceptor stimulation[J]. Cardiovasc Res, 2000, 45(1): 163–171. |
| [6] | LIU X H, ZHANG Q, HAN M, et al. Intrapericardial capsaicin and bradykinin induce different cardiac-somatic and cardiovascular reflexes in rats[J]. Auton Neurosci, 2016, 198: 28–32. DOI:10.1016/j.autneu.2016.06.001 |
| [7] | CRISAFULLI A. The Impact of cardiovascular diseases on cardiovascular regulation during exercise in humans:Studies on metaboreflex activation elicited by the post-exercise muscle ischemia method[J]. Curr Cardiol Rev, 2017, 13(4): 293–300. |
| [8] | CATERINA M J, SCHUMACHER M A, TOMINAGA M, et al. The capsaicin receptor:a heat-activated ion channel in the pain pathway[J]. Nature, 1997, 389(6653): 816–824. DOI:10.1038/39807 |
| [9] | QUARTU M, SERRA M P, BOI M, et al. TRPV1 receptor in the human trigeminal ganglion and spinal nucleus:immunohistochemical localization and comparison with the neuropeptides CGRP and SP[J]. J Anat, 2016, 229(6): 755–767. DOI:10.1111/joa.12529 |
| [10] | AGHAZADEH TABRIZI M, BARALDI P G, BARALDI S, et al. Medicinal chemistry, pharmacology, and clinical implications of TRPV1 receptor antagonists[J]. Med Res Rev, 2017, 37(4): 936–983. DOI:10.1002/med.21427 |
| [11] | HERMES S M, ANDRESEN M C, AICHER S A. Localization of TRPV1 and P2X3 in unmyelinated and myelinated vagal afferents in the rat[J]. J Chem Neuroanat, 2016, 72: 1–7. DOI:10.1016/j.jchemneu.2015.12.003 |
| [12] | MOHAMMED M, MADDEN C J, ANDRESEN M C, et al. Activation of TRPV1 in nucleus tractus solitarius reduces brown adipose tissue thermogenesis, arterial pressure, and heart rate[J]. Am J Physiol Regul Integr Comp Physiol, 2018, 315(1): :R134–R143. DOI:10.1152/ajpregu.00049.2018 |
| [13] | HE B, LU Z B, HE W B, et al. Autonomic modulation by electrical stimulation of the parasympathetic nervous system:An emerging intervention for cardiovascular diseases[J]. Cardiovasc Ther, 2016, 34(3): 167–171. DOI:10.1111/1755-5922.12179 |
| [14] | BRAUNWALD E. The war against heart failure:the Lancet lecture[J]. Lancet, 2015, 385(9970): 812–824. DOI:10.1016/S0140-6736(14)61889-4 |
| [15] | GAO YF, SONG JX, CHEN H, et al. TRPV1 activation is involved in the cardioprotection of remote limb ischemic postconditioning in ischemia-reperfusion injury rats[J]. Biochem Biophys Res Commun, 2015, 463(4): 1034–1039. DOI:10.1016/j.bbrc.2015.06.054 |
| [16] | ICHIGE M H, SANTOS C R, JORDÃO C P, et al. Exercise training preserves vagal preganglionic neurones and restores parasympathetic tonus in heart failure[J]. J Physiol(Lond), 2016, 594(21): 6241–6254. |
| [17] | ZHANG J H, WU Z P, FAN ZP, et al. Pericardial application as a new route for implanting stem-cell cardiospheres to treat myocardial infarction[J]. J Physiol(Lond), 2018, 596(11): 2037–2054. |
| [18] | 冯丹阳, 孙强, 陈莉娜, 等. Ghrelin对糖尿病大鼠心肌动作电位和瞬时外向钾电流的影响[J]. 西安交通大学学报:医学版, 2017, 38(5): 661–664. |
| [19] | LI J, ZHANG M M, TU K, et al. The excitatory synaptic transmission of the nucleus of solitary tract was potentiated by chronic myocardial infarction in rats[J]. PLoS One, 2015, 10(3): e0118827. DOI:10.1371/journal.pone.0118827 |
| [20] | HUA F, HARRISON T, QIN C, et al. C-Fos expression in rat brain stem and spinal cord in response to activation of cardiac ischemia-sensitive afferent neurons and electrostimulatory modulation[J]. Am J Physiol Heart Circ Physiol, 2004, 287(6): H2728–H2738. DOI:10.1152/ajpheart.00180.2004 |
| [21] | LIU X H, HAN M, ZHU J X, et al. Metabotropic glutamate subtype 7 and 8 receptors oppositely modulate cardiac nociception in the rat nucleus tractus solitaris[J]. Neuroscience, 2012, 220: 322–329. DOI:10.1016/j.neuroscience.2012.05.024 |
 2019, Vol. 45
2019, Vol. 45


