扩展功能
文章信息
- 唐庚, 史耀庭, 孔维轩, 柯希扬, 李成想, 邱禹淇, 于雷, 杨艳明, 王志成
- TANG Geng, SHI Yaoting, KONG Weixuan, KE Xiyang, LI Chengxiang, QIU Yuqi, YU Lei, YANG Yanming, WANG Zhicheng
- 电离辐射对沉默ATRX的HeLa细胞DNA损伤修复的影响
- Effect of ionizing radiation on DNA damage repair in HeLa cells silencing ATRX
- 吉林大学学报(医学版), 2019, 45(05): 981-985
- Journal of Jilin University (Medicine Edition), 2019, 45(05): 981-985
- 10.13481/j.1671-587x.20190501
-
文章历史
- 收稿日期: 2019-01-03
2. 黑龙江省农垦总局总医院肿瘤四科, 黑龙江 哈尔滨 150088;
3. 吉林大学第二医院放疗科, 吉林 长春 130041
2. Department of Oneology, General Hospital, Heilongjiang Province Land Reclamation Bureau, Haerbin 150088, China;
3. Department of Radiotherapy, Second Hospital, Jilin University, Changchun 130041, China
α-地中海贫血/精神发育迟滞综合征X染色体相关蛋白(alpha thalassemia/mental retardation syndrome X-linked protein, ATRX)属于SWI2/SNF2家族成员,具有广泛的生物学功能,参与DNA损伤修复、转录调节和染色质重组等[1-2]ATRX主要定位于细胞富含CCCTAA的端粒末端,且与G-四联体相互作用,是一个重要的DNA二级结构,可能导致DNA复制应激[3-4]。ATRX是一种肿瘤抑制因子,在许多肿瘤细胞中往往也伴随着ATRX的缺失,如U2OS细胞、脑胶质细胞瘤和脂肪肉瘤等[5-6]。肿瘤基因-放射治疗是辐射肿瘤学相关研究的热点,辐射可致DNA损伤,进而引发肿瘤细胞凋亡或坏死,达到治疗肿瘤的目的。DNA损伤方式主要包括单链断裂(single strand breaks,SSB)、双链断裂(double strand break,DSB)、碱基的修饰和丧失碱基位点等,其中DSB最严重[7]。细胞在电离辐射作用后,H2AX在极短时间内迅速磷酸化γH2AX并在DSB位点形成焦点,而后者所形成焦点数量与电离辐射造成的DSB数量存在对应关系[8-9]。DSB修复的主要通路包括同源重组修复(homologous recombination,HR)和非同源末端连接(non-homologous end joining,NHEJ),二者在损伤修复时涉及到多种信号通路。Rad51蛋白酶是HR的关键酶、损伤的感应器和周期检查点关键蛋白及体内催化同源重组性DNA修复最主要的关键酶[10]。因此,本研究通过建立稳定靶向沉默ATRX的HeLa细胞模型,并给予电离辐射,分别检测ATRX、γH2AX和RAD51蛋白表达以及γH2AX和RAD51焦点的形成,探讨ATRX参与辐射诱导HeLa细胞DNA损伤修复的作用,为肿瘤放射治疗提供新的理论和实验依据。
1 材料与方法 1.1 细胞、试剂和主要仪器人宫颈癌HeLa细胞和293T细胞由本实验室保存。MEM培养基和胎牛血清(美国Gibco公司),HieffTransTM脂质体核酸转染试剂(上海翊圣生物科技有限公司),青链霉素(美国ThermoFisher scientific公司),GAPDH、ATRX、γH2AX和Rad51一抗(美国Santa Cruz公司),Red fluorescent抗兔二抗(美国CST公司),辣根过氧化物酶标记的二抗(美国Immunoway公司),嘌呤毒素puromycin(美国Sigma公司),其他试剂为国产。X射线辐照仪X-RAD 320iX(Precision X-ray,美国Inc公司),垂直板电泳系统(美国BioRad公司)。
1.2 靶向沉默ATRX的HeLa细胞的获得靶向ATRX的3段sh RNA序列分别为5′-ATCCTCAAGAGGTTGAATC-3′、5′-TTTCTTATGTTCACCACCG-3′和5′-TTATCTTGTGGAACTTCCT-3′。分别构建到pGIPz载体上,即pGIPz-shATRX1、pGIPz-shATRX2和pGIPz-shATRX3,同时设立pGIPz-shControl为阴性对照,pSPAX2和pMD2G质粒(美国罗格斯大学肿瘤研究所沈智渊博士惠赠)。利用Hieff TransTM转染试剂将pGIPz-shControl、pGIPz-shATRX1、pGIPz-shATRX2和pGIPz-shATRX3与pSPAX2和pMD2G分别共转染293T细胞,Hieff TransTM:shRNA:pSPAX2:pMD2G=120 μL:3 μg:1.5 μg:1.5 μg。48和72 h收取上清液并利用0.45 μm滤膜过滤后加到培养于6孔板的HeLa细胞中,共感染2次。通过观察绿色荧光状态判定感染效率,并加入10 μL的puromycin进行阳性筛选,命名为shCon-HeLa、shA1-HeLa、shA2-HeLa和shA3-HeLa细胞,逐渐将阳性细胞扩大并冻存液氮中备用。
1.3 Western blotting法检测蛋白表达分别将shCon-HeLa、shA1-HeLa、shA2-HeLa和shA3-HeLa细胞接种于6孔板,按照每孔1×107个细胞,24 h后收集细胞,并加入裂解液RIPA 100 μL,超声后加入5×loading buffer,100℃变性10 min后,冷却样品直接上样;取shCon-HeLa和shA1-HeLa组细胞进行2和8 Gy X射线照射,1、6和24 h后提取总蛋白;另取上述2组细胞进行4 Gy照射,0、0.5、1.0、3.0和6.0 h提取总蛋白;40 μg蛋白变性后上样,浓缩胶80 V,分离胶120 V,SDS-PAGE电泳后转膜缓冲液4℃中过夜湿转,5%脱脂奶粉封闭1 h后,ATRX一抗(TBST配置,1:1 000)、GAPDH、γH2AX和Rad51一抗(1:500),37℃孵育2 h,TBST洗3次,每次10 min,加入辣根过氧化物酶标记的二抗(TBST配置,1:3 000)后37℃孵育1 h,TBST洗3次,加入ECL液A和B,暗室中曝光,拍照分析。
1.4 免疫荧光技术检测γH2AX和Rad51焦点数分别将shCon-HeLa和shA1-HeLa细胞接种于放置了盖玻片的6孔板,按照每孔1×105个细胞,12 h后采用4 Gy照射,照射后分别于0、0.5、1.0、3.0和6.0h采用4%多聚甲醛固定10 min,2%BSA + 0.3% TritonX100封闭液室温封闭1 h,γH2AX一抗和Rad51一抗(1:500)4℃过夜孵育,PBS洗5次,每次5 min,红色荧光二抗(1:1 000)室温孵育1 h,PBS洗5次,每次5 min,8 μL的DAPI滴加到载玻片上,将带有细胞的盖玻片放置在载玻片上进行封片。荧光显微镜下观察γH2AX和Rad51焦点形成(点状红色荧光所示),随机选取5个视野,每个视野选择20个细胞,计数焦点数。
1.5 统计学分析采用SPSS 24.0统计软件进行统计学分析。γH2AX和Rad51焦点数以x±s表示,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 Western blotting法检测ATRX蛋白表达shCon-HeLa细胞中可见ATRX蛋白表达,而shA1-HeLa、shA2-HeLa和shA3-HeLa细胞中无ATRX蛋白表达,表明靶向沉默ATRX的HeLa细胞模型构建成功。见图 1。
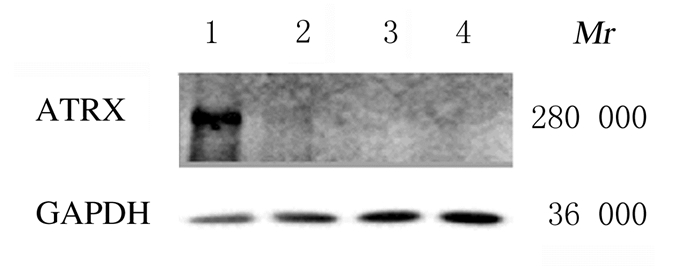
|
| Lane 1: shCon-HeLa cells; Lane 2: shA1-HeLa cells; Lane3: shA2-HeLa cells; Lane 4: shA3-HeLa cells. 图 1 Western blotting法检测4种细胞模型中ATRX蛋白表达电泳图 Fig. 1 Electrophoregram of ATRX protein expressions in 4 kinds of cell models measured by Western blotting method |
|
|
shCon-HeLa组和shA1-HeLa组细胞给予2和8 GyX射线照射,分别于1、6和24 h检测ATRX蛋白表达。2 Gy照射后,shCon-HeLa细胞中ATRX蛋白表达量随照射后时间延长明显增加,24 h时达到最大值;而8 Gy照射后,ATRX蛋白表达量一直处于较高水平。见图 2。
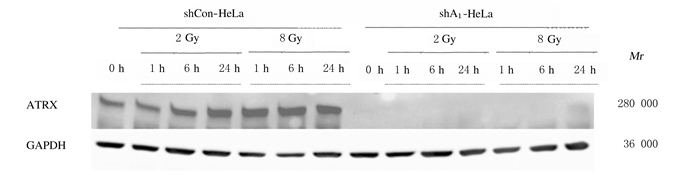
|
| 图 2 2和8Gy X射线照射后1、6和24 h时ATRX蛋白表达电泳图 Fig. 2 Electrophoregramof ATRX protein expressions at 1, 6, and 24 h after 2 and 8 Gy X-ray irradiation |
|
|
荧光显微镜下观察4 Gy照射后shCon-HeLa组和shA1-HeLa组细胞中γH2AX和Rad51焦点形成:0~6.0 h时shCon-HeLa组和shA1-HeLa组细胞中γH2AX和Rad51焦点数均在1.0 h时最多,而后逐渐降低,与shCon-HeLa组比较,shA1-HeLa组细胞中γH2AX和Rad51焦点数在1.0和6.0 h均明显增多(P < 0.05或P < 0.01)。见表 1和图 3(插页一)。

|
| 图 3 荧光显微镜观察4 Gy照射后0~6 h时2组细胞中γH2AX和Rad51焦点形成~ Fig. 3 Formation of γH2AX and Rad51 foci in cells in two groups 0-6 h after 4 Gy irradiation observed by fluorescence microscope |
|
|
| (n=100, x ±s) | |||||
| Group | γH2AX foci | ||||
| (t/h) 0 | 0.5 | 1.0 | 3.0 | 6.0 | |
| shCon-HeLa | 2.26±0.76 | 27.73±4.94 | 41.00±2.91 | 25.20±8.42 | 14.20±5.04 |
| shA1-HeLa | 2.31±0.35 | 28.80± 6.18 | 46.91±2.19* | 29.73±6.64 | 23.40±5.65** |
| Group | Rad51 foci | ||||
| (t/h) 0 | 0.5 | 1.0 | 3.0 | 6.0 | |
| shCon-HeLa | 2.12±0.93 | 7.03±1.57 | 16.27±3.60 | 17.37±3.76 | 6.50±1.61 |
| shA1-HeLa | 2.11±1.05 | 7.47±2.07 | 19.33±3.59* | 19.23±3.48 | 18.16±4.96** |
| * P < 0.05, * * P < 0.01 compared with shCon-HeLa group. | |||||
4 GyX射线照射后1.0和6.0 h,shA1-HeLa组细胞中Rad51和γH2AX蛋白表达量均高于shCon-HeLa组,与γH2AX和Rad51焦点数的变化规律基本一致。见图 4。
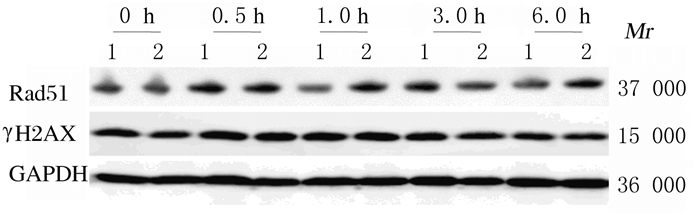
|
| Lane1: shCon-HeLagroup; Lane 2: shA1-HeLa group. 图 4 Western blotting法检测4 Gy X射线照射后不同时间点γH2AX和Rad51蛋白表达电泳图 Fig. 4 Electrophoregram of expressions of γH2AX and Rad51 proteins at different time points after 4 Gy X-ray irradiation detected by Western blotting method |
|
|
DNA损伤修复涉及非常多的基因,ATRX基因是一种重要的DNA损伤修复基因,其主要功能包括维持端粒的稳定、染色体黏附、维持DNA结构和直接连接到双链断裂位点等[11-14]。放射治疗杀伤肿瘤细胞的基本机制是DNA的损伤修复反应,因放射治疗会造成基因组DNA的DSB,导致细胞DNA损伤后得不到正确的修复,引起细胞发生凋亡,进而使细胞丧失增殖能力[15]。本研究利用靶向ATRX基因的慢病毒shRNA表达载体,转染293T细胞后,利用慢病毒2次感染宫颈癌HeLa细胞,并经puromycin阳性筛选获得稳定ATRX沉默的细胞模型,Western blotting法检测结果显示:3条靶向ATRX的序列具有较好的沉默效果,均可作为候选细胞进行后续研究,故选取shCon-HeLa和shA1-HeLa细胞进行后续研究。电离辐射作为一种高能物理损伤因素,可以通过直接或间接作用引起受照射生物组织和细胞的损伤。在分子水平上,无论是辐射的直接作用还是间接作用产生的自由基和生物大分子(脂质、蛋白质、DNA和RNA等)均是辐射的直接效应靶点。在生命进程中,DNA难免会受到源于外界环境中有害的化学物质、紫外线以及射线等威胁,所造成的DNA损伤也是正常细胞转变为肿瘤细胞的主要诱因之一。电离辐射产生的DNA损伤穿透力强,不受亚细胞结构的影响,发生速度极快,而且损伤的形式多样,包含碱基和核苷酸水平的损伤以及链损伤。辐射导致的DNA损伤包括SSB和DSB、碱基损伤和DNA-蛋白交联[16-17]。DSB是辐射后严重的损伤,主要通过HR和NHEJ修复[18-19]。2和8 Gy照射后,shCon-HeLa细胞中ATRX蛋白表达量明显增加,提示其参与辐射导致的DNA损伤修复。γH2AX和Rad51焦点及蛋白表达均可作为DSB修复的标志[8-10],因此本实验检测二者焦点数及蛋白表达,以反映辐射后DNA损伤修复的状态。4 Gy X射线照射后shCon-HeLa和shA1-HeLa细胞中γH2AX和Rad51焦点数均迅速增高,在1 h时达到最大值,至6 h时逐渐降低,但仍高于0 h;与shCon-HeLa组比较,shA1-HeLa组细胞中γH2AX和Rad51焦点数均明显升高,提示缺失ATRX基因后,HR修复能力降低,ATRX参与DNA损伤后的HR修复。采用Western blotting法检测2种蛋白表达结果显示:在2种细胞间存在γH2AX和Rad51蛋白表达的差异,与焦点形成数的规律基本一致。上述结果显示:辐射损伤DNA后ATRX参与双链断裂的修复,是一个重要的HR修复的参与者。
综上所述,本研究利用靶向ATRX的慢病毒实现了沉默HeLa细胞中ATRX的目的,经过电离辐射后,对照组ATRX具有辐射增强表达的特性,且对照组和缺失ATRX的HeLa细胞中γH2AX和Rad51焦点数增加,随时间延长对照组逐渐回落,而缺失ATRX的HeLa细胞中焦点数降低则较慢,且蛋白表达的结果与其相似,提示缺失ATRX后细胞修复能力降低。以ATRX为靶点的肿瘤基因-放射治疗为临床放射治疗提供了新的理论和实验数据。
| [1] | MITSON M, KELLEY L A, STERNBERG M J, et al. Functional significance of mutations in the Snf2 domain of ATRX[J]. Hum Mol Genet, 2011, 20(13): 2603–2610. DOI:10.1093/hmg/ddr163 |
| [2] | KOSCHMANN C, CALINESCU A A, NUNEZ F J, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma[J]. Sci Transl Med, 2016, 8(328): 328ra28. DOI:10.1126/scitranslmed.aac8228 |
| [3] | LAW M J, LOWER K M, VOON H P, et al. ATR-X syndrome protein targets tandem repeats and I nfluences allele-specific expression in a size-dependent manner[J]. Cell, 2010, 143(3): 367–378. DOI:10.1016/j.cell.2010.09.023 |
| [4] | DUC C, BENOIT M, DÉTOURN G, et al. Arabidopsis ATRX modulates H3.3 occupancy and fine-tunes gene expression[J]. Plant Cell, 2017, 29(7): 1773–1793. DOI:10.1105/tpc.16.00877 |
| [5] | MUKHERJEE J, JOHANNESSEN T C, OHBA S, et al. Mutant IDH1 cooperates with ATRX loss to drive the alternative lengthening of telomere phenotype in glioma[J]. Cancer Res, 2018, 78(11): 2966–2977. DOI:10.1158/0008-5472.CAN-17-2269 |
| [6] | VUONG H G, TRAN T T K, NGO H T T, et al. Prognostic significance of genetic biomarkers in isocitrate dehydrogenase-wild-type lower-grade glioma:the need to further stratify this tumor entity-a meta-analysis[J]. Eur J Neurol, 2019, 26(3): 379–387. DOI:10.1111/ene.13826 |
| [7] | 龚守良. 医学放射生物学[M]. 4版.北京: 中国原子能出版社,2015. |
| [8] | KUEFNER M A, BRAND M, ENGERT C, et al. Radiation induced DNA double-strand breaks in radiology[J]. Rofo, 2015, 187(10): 872–878. DOI:10.1055/s-0035-1553209 |
| [9] | CAMERO S, CECCARELLI S, DE FELICE F, et al. PARP inhibitors affect growth, survival and radiation susceptibility of human alveolar and embryonal rhabdomyosarcoma cell lines[J]. J Cancer Res Clin Oncol, 2019, 145(1): 137–152. DOI:10.1007/s00432-018-2774-6 |
| [10] | LILLIAN C D, SALAHUDDIN S, KRISTINA H S. Sgs1 binding to Rad51 stimulates homology-directed DNA repair in Saccharomyces cerevisiae[J]. Genetics, 2018, 208(1): 125–138. DOI:10.1534/genetics.117.300545 |
| [11] | VOON H P, HUGHES J R, RODE C, et al. ATRX plays a key role in maintaining silencing at interstitial heterochromatic loci and imprinted genes[J]. Cell Rep, 2015, 11(3): 405–418. DOI:10.1016/j.celrep.2015.03.036 |
| [12] | NOH K M, MAZE I, ZHAO D, et al. ATRX tolerates activity-dependent histone H3 methyl/phos switching to maintain repetitive element silencing in neurons[J]. Proc Natl Acad Sci U S A, 2015, 112(22): 6820–6827. DOI:10.1073/pnas.1411258112 |
| [13] | DE LA FUENTE R, BAUMANN C, VIVEIROS M M. Chromatin structure and ATRX function in mouse oocytes[J]. Results Probl Cell Differ, 2012, 55: 45–68. |
| [14] | RATNAKUMAR K, BERNSTEIN E. ATRX:the case of a peculiar chromatin remodeler[J]. Epigenetics, 2013, 8(1): 3–9. |
| [15] | LUO J, SI Z Z, LI T, et al. MicroRNA-146a-5p enhances radiosensitivity in hepatocellular carcinoma through replication protein A3 induced activation of the DNA repair pathway[J]. Am J Physiol Cell Physiol, 2019, 316(3): C299–C311. DOI:10.1152/ajpcell.00189.2018 |
| [16] | NAKANO T, XU X, SALEM A M H, et al. Radiation-induced DNA-protein cross-links:Mechanisms and biological significance[J]. Free Radic Biol Med, 2017, 107: 136–145. DOI:10.1016/j.freeradbiomed.2016.11.041 |
| [17] | SAGE E, SHIKAZONO N. Radiation-induced clustered DNA lesions:Repair and mutagenesis[J]. Free Radic Biol Med, 2017, 107: 125–135. DOI:10.1016/j.freeradbiomed.2016.12.008 |
| [18] | 赵忆宁, 何颖, 沈先荣, 等. 西咪替丁对低剂量电离辐射致大鼠氧化应激的保护作用[J]. 解放军医学杂志, 2017, 42(2): 128–133. |
| [19] | CECCALDI R, RONDINELLI B, D'ANDREA A D. Repair pathway choices and consequences at the double-strand break[J]. Trends Cell Biol, 2016, 26(1): 52–64. DOI:10.1016/j.tcb.2015.07.009 |
 2019, Vol. 45
2019, Vol. 45


