扩展功能
文章信息
- 郑天航, 张君, 郑柏松
- ZHENG Tianhang, ZHANG Jun, ZHEN Baisong
- 人骨髓基质干细胞抗原2在肿瘤发生发展中作用的研究进展
- Research progress in role of human bone marrow stromal antigen-2 in occurrence and development of tumor
- 吉林大学学报(医学版), 2019, 45(04): 976-980
- Journal of Jilin University (Medicine Edition), 2019, 45(04): 976-980
- 10.13481/j.1671-587x.20190442
-
文章历史
- 收稿日期: 2018-06-20
宿主为抵御外源病毒的攻击而进化出一系列防御机制,包括天然免疫系统等。天然抗病毒因子作为人体内重要的对抗外源入侵的手段,已成为人体天然防御的重要组成部分。随着研究的深入,目前公认的抗病毒因子包括载脂蛋白B-mRNA编辑酶复合物3(apolipoprotein B mRNA editing enzyme-catalytic polypeptide-like 3,APOBEC3)[1]、天然免疫蛋白—包含SAM和HD结构域蛋白1(SAM domain and HD domain 1,SAMHD1)[2]、人骨髓基质干细胞抗原2(bone marrow stromal antigen-2,BST-2)[3]和丝氨酸整合因子(serine incorporalor,SERINC)[4]等。在发现BST-2为一种有效的天然抗病毒因子之前,BST-2一直被认为是骨髓基质干细胞和成熟B细胞的表面标记物,同时是一种在多种多发性骨髓瘤细胞中高表达的肿瘤抗原[5-6]。BST-2抗体一度被用于多发性骨髓瘤的免疫治疗。组织微阵列的表达谱研究[7]显示:BST-2在人肝细胞、肾细胞和上皮细胞,特别是肺和胃肠道的分泌组织中均有表达。BST-2既是病毒限制因子又是促癌因子,国内外均有很多文献报道了其对艾滋病病毒(HIV)、乙型肝炎病毒(HBV)、肠道病毒71型(EV71)和流感病毒等的限制作用[8-11],但是国内外关于BST-2对于肿瘤发生发展影响的综述类报道比较少,本文作者对BST-2促进癌症进展的作用机制进行综述。
1 BST-2分子结构和功能BST-2是由干扰素诱导产生的单次跨膜Ⅱ型膜蛋白[12-14],由180个氨基酸组成,蛋白相对分子质量为28000~36000,具有比较特殊的拓扑结构[6]。BST-2通常在树突状细胞、终末分化的B细胞和骨髓基质细胞中能检测到[15]。BST-2结构见图 1。
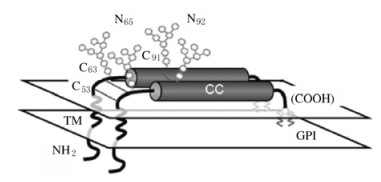
|
| 图 1 BST-2结构图 Fig. 1 Structure diagram of BST-2 |
|
|
BST-2包括4个区域,分别是N-末端胞质尾段(CT)、跨膜区(TM)、胞外螺旋区和C-末端糖基磷脂酰肌醇(GPI)锚定位点[16-18]。BST-2胞外段区域的第53、63和91位氨基酸为半胱氨酸,可通过形成分子间二硫键,介导BST-2单体形成同源二聚体;第65和92位氨基酸为天冬酰胺,介导BST-2形成N-链接糖基化。BST-2主要定位于细胞膜(PM)、内吞小体和高尔基体(TGN)中,其胞内段含有一段保守的双重酪氨酸序列,涉及到BST-2与网格蛋白适配蛋白AP1和AP2结合,并介导BST-2的内吞循环。GPI锚定位点对于新合成的BST-2蛋白从内质网(ER)转运到细胞表面是必需的[19]。
BST-2作为一种有效的病毒限制因子,通过GPI锚定,将包膜病毒系到细胞膜上,进而有效地抑制病毒在体外细胞和体内中的复制。BST-2被认为通过其胞质结构域上的YXY基序与转化生长因子β激活激酶1(TAK1)和TNF受体相关因子(TRAF)2、TRAF6相互作用,激活NF-κB介导的宿主免疫应答,增加了可能抑制病毒复制的炎症介质,可以作为有效的NF-κB激活剂[20-21]。尽管过表达的BST-2能够将病毒颗粒系在细胞膜上,并负调节病毒的复制,但BST-2表达的升高可能会正向调节癌细胞行为。研究[22]表明:BST-2对乳腺癌、口腔癌、鼻咽癌、胃肠癌和肺癌等癌细胞的生长和转移产生影响。
2 BST-2对肿瘤的影响 2.1 BST-2对乳腺癌的影响研究[23]显示:在部分浸润性原发性乳腺癌中,可观察到BST-2相对丰富的表达及其在宿主感染的免疫细胞的病毒释放中发挥抑制作用;BST-2抗体介导的抗体依赖的细胞介导的细胞毒作用(antibody-dependent cell-mediated cytotoxicity,ADCC)在骨髓瘤治疗和乳腺癌治疗中有效,并且BST-2在驱动乳腺恶性肿瘤中起直接作用,对乳腺癌细胞的侵袭性和体内转移的形成至关重要。在体内,BST-2表达水平升高与原发性肿瘤生长、转移和预后不良有关。敲除BST-2后,乳腺癌细胞失去了在体内生长和繁殖的能力。研究[24]显示:BST-2在乳腺肿瘤中的表达水平与肿瘤大小、肿瘤侵袭性和宿主生存能力有关联。体外实验[25]显示:将敲除BST-2的细胞接种到BALB/c小鼠的乳房脂肪垫中评估肿瘤生长,敲除组小鼠乳腺肿瘤潜伏期延长,乳腺肿瘤发作延迟,且肿瘤体积随时间增长而减小,说明在乳腺癌细胞中抑制BST-2表达可延长原发性肿瘤形成的时间,减轻肿瘤的质量,并减少肿瘤的转移;通过荧光染色发现其可促进癌细胞与成纤维细胞的黏附,从而促进癌细胞迁移和侵袭。
2.2 BST-2对口腔癌的影响FANG等[14]发现:BST-2是与口腔鳞状细胞癌(OSCC)转移相关的潜在分子之一。BST-2在OSCC肿瘤组织中过表达,高BST-2表达与几种OSCC临床病理学表现有关联,包括阳性N期、整体晚期、神经浸润和肿瘤深度,这些现象说明BST-2与OSCC肿瘤进展和转移有关。此外,BST-2的RNA干扰实验证实了BST-2的阻断能够减弱体外OSCC细胞的迁移。阻断BST-2及其相关途径可能对未来OSCC治疗起到潜在的作用。
2.3 BST-2对鼻咽癌的影响近年来,鼻咽癌(NPC)的发病率呈上升趋势[26]。基于顺铂的化疗被认为是局部晚期NPC的标准治疗方案。BST-2在顺铂敏感的NPC细胞系中下调,在鼻咽癌细胞中敲减BST-2可敏化对顺铂的反应,并促进顺铂诱导的细胞凋亡;而外源性BST-2的过度表达可增加顺铂耐药性,并抑制顺铂诱导的细胞凋亡。高水平BST-2可能是铂类化疗治疗局部晚期鼻咽癌患者预后不良的独立指标。NPC是EB病毒(EBV)相关的恶性肿瘤,研究[27]显示:包括BST-2在内的一类干扰素刺激基因(ISG)在Ⅲ型NPC肿瘤中明显活化。同时,内源性EBV复制在Ⅲ型NPC中被抑制,从而使病毒潜伏。因此,推测BST-2表达升高可能是由Ⅲ型NPC潜伏EBV感染引起的,BST-2可能在EBV潜伏期、肿瘤发生和顺铂耐药中具有多种功能[27-28]。上述研究结果提示:BST-2可能为NPC患者个性化和精确治疗策略制定提供指导。
2.4 BST-2对肺癌的影响肺癌是世界范围内导致癌症死亡的主要原因[29-30]。基因表达系列分析(SAGE)和微阵列分析方法研究[31]显示:BST-2基因在几种实体肿瘤细胞中过表达,且肿瘤细胞表现出侵袭性或耐药性表型。在42%的肺癌细胞系以及来自恶性胸膜腔积液原代培养的肺癌细胞中表达BST-2抗原,并且抗BST-2抗体的使用明显抑制了肺癌细胞的生长[13],表明BST-2是一种基于抗体以及疫苗研究用于治疗肺癌的免疫学靶点。
3 BST-2影响肿瘤发生发展的机制BST-2对包括乳腺癌、胃肠癌、肺癌和鼻咽癌等在内的多种不同癌症进程具有促进作用。有研究[33]揭示了TGF-β通路调控BST-2的分子机制;研究[34-35]显示:甲基化形式的BST-2对肿瘤进程有明显的影响,且揭示了二聚化形式的BST-2对于肿瘤生存影响的分子机制。BST-2影响肿瘤进程具体机制的阐明,为癌症的研究及治疗提供了理论依据及潜在方法。
3.1 BST-2甲基化影响肿瘤进程表观遗传学改变和基因功能的调节是致癌作用的关键[36-37],其可能涉及组蛋白修饰和CpG位点(胞嘧啶-磷酸-鸟嘌呤位点)二核苷酸背景下胞嘧啶碱基(C)的DNA甲基化状态的改变。对癌症基因组图谱(TCGA)和各种基因表达综合数据集(GEO)中BST-2基因表达及其DNA甲基化进行Meta分析,比较各种乳腺肿瘤分子亚型和乳腺癌细胞系中BST-2基因特定CpG位点的BST-2表达水平和BST-2 DNA甲基化状态,在乳腺癌组织和细胞中观察到更高频率的BST-2低甲基化[34]。研究[38-39]显示:在癌症基因组中,DNA超甲基化发生在肿瘤抑制基因的启动子区域,可能导致肿瘤抑制基因的沉默。相反,DNA低甲基化常常发生在DNA重复中,导致基因组不稳定和癌症基因组中的突变。因此,DNA低甲基化的BST-2过表达可促进乳腺癌发生,并可预测乳腺癌预后或治疗反应。
3.2 BST-2二聚化影响肿瘤进程临床乳腺癌模型[24]验证了BST-2二聚化在体外和体内促进肿瘤生长的机制:通过蛋白酶体介导的前凋亡因子BIM-α的降解,位于BST-2细胞外结构域(ECD)中的半胱氨酸残基的二聚化导致了凋亡抵抗。研究[40]表明:BST-2二聚化的破坏可防止乳腺癌细胞彼此黏附。此外,ECD区域半胱氨酸残基的恢复可通过BST-2 /BIM途径促进细胞存活和肿瘤生长[24]。BST-2二聚化的破坏为癌症提供了潜在的治疗方法。
3.3 BST-2通过TGF-β调控肿瘤发生TGF-β家族成员是在多种细胞类型中发挥一系列生物效应的多能细胞因子。研究[33]显示:TGF-β途径与癌症的病理生理学有密切关联。TGF-β可作为一种有效的肿瘤抑制因子[41-42]。在乳腺癌中,BST-2受到TGF-β的负调控,转录因子AP2是TGF-β介导的乳腺癌BST-2调控中的重要组成部分,抑制TGF-β通路后,转录因子AP2与BST-2启动子的结合减弱,从而增加肿瘤细胞中BST-2的表达。
3.4 BST-2参与信号传导转录激活因子3(STAT3)途径调控肿瘤发生肿瘤细胞中STAT3的激活可以促进细胞因子诱导的肿瘤生长;同时,激活的STAT3可以调节细胞因子的产生从而抑制肿瘤特异的免疫应答,并且诱导肿瘤转移[43-44]。BST-2的串联重复序列含有3个STAT3结合基序,位于转录起始位点的146和126之间,表明STAT3可能参与了BST-2的表达[45],且STAT3可能通过增强乳腺癌中BST-2的启动和表达促进肿瘤细胞的侵袭、迁移和转移。在口腔癌和乳腺癌中,BST-2可能通过STAT3/ BST2 /白细胞介素6(IL-6)通路在肿瘤细胞的侵袭和转移中发挥作用[5]。因此,BST-2是肿瘤侵袭性和转移的重要因素,并可能成为治疗转移性和化疗耐药性口腔癌及乳腺癌患者新方法的新靶点。
3.5 BST-2通过核因子κB(NF-κB)信号通路调控肿瘤发生研究[46]表明:在BST-2过表达的鼻咽癌细胞系中总,NF-κB抑制因子α(IκBα)减少,磷酸化IκBα(p-IκBα)和p65增加,BST-2介导的顺铂耐药性依赖于NF-κB信号通路的激活以及抗凋亡基因(如Bcl-XL和livin)的上调。此外,在鼻咽癌患者和裸鼠的癌组织中,BST-2蛋白水平与Bcl-XL水平呈正相关关系,与IκBα水平呈负相关系。GALAO等[46]报道:过表达BST-2 Y6, 8A突变体并不增加鼻咽癌细胞中的铂类耐药性,而过表达ΔGPI突变体和野生型BST-2对铂类耐药性具有相似的作用,证实这些突变体可影响NF-κB的激活。
4 总结与展望目前,针对BST-2与肿瘤关系的研究还很少,前期研究显示BST-2在多种肿瘤组织中高表达,对肿瘤的发生、侵袭和转移发挥重要作用,初步揭示了BST-2的表达、转录后修饰及参与信号通路调控肿瘤发生发展的不同分子机制,是否可通过其他机制发挥促肿瘤作用,尚待进一步探索。现已明确BST-2在肿瘤诊治方面具有重要价值,可为肿瘤的诊治提供新靶点和思路。
| [1] | EBRAHIMI D, RICHARDS CM, CARPENTER MA, et al. Genetic and mechanistic basis for APOBEC3H alternative splicing, retrovirus restriction, and counteraction by HIV-1 protease[J]. Nat Commun, 2018, 9(1): 4137. DOI:10.1038/s41467-018-06594-3 |
| [2] | HRECKA K, HAO CL, GIERSZEWSKA M, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein[J]. Nature, 2011, 474(7353): 658–661. DOI:10.1038/nature10195 |
| [3] | SONG YE, CYBURT D, LUCAS TM, et al. βTrCP is required for HIV-1 Vpu modulation of CD4, GaLV Env, and BST-2/Tetherin[J]. Viruses, 2018, 10(10): E573. DOI:10.3390/v10100573 |
| [4] | CHANDE A, CUCCURULLO E C, ROSA A, et al. S2 from equine infectious anemia virus is an infectivity factor which counteracts the retroviral inhibitors SERINC5 and SERINC3[J]. Proc Natl Acad Sci U S A, 2016, 113(46): 13197–13202. DOI:10.1073/pnas.1612044113 |
| [5] | OHTOMO T, SUGAMATA Y, OZAKI Y, et al. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells[J]. Biochem Biophys Res Commun, 1999, 258(3): 583–591. DOI:10.1006/bbrc.1999.0683 |
| [6] | ISHIKAWA J, KAISHO T, TOMIZAWA H, et al. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth[J]. Genomics, 1995, 26(3): 527–534. DOI:10.1016/0888-7543(95)80171-H |
| [7] | ERIKSON E, ADAM T, SCHMIDT S, et al. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans[J]. Proc Natl Acad Sci U S A, 2011, 108(33): 13688–13693. DOI:10.1073/pnas.1101684108 |
| [8] | SINGH H, SAMANI D, GHATE M V, et al. Impact of cellular restriction gene (TRIM5α, BST-2) polymorphisms on the acquisition of HIV-1 and disease progression[J]. J Gene Med, 2018, 20(2/3): e3004. |
| [9] | WANG M Y, ZHANG Z Y, WANG X J. Strain-specific antagonism of the human H1N1 influenza A virus against equine tetherin[J]. Viruses, 2018, 10(5): E264. DOI:10.3390/v10050264 |
| [10] | HAN Z, LV M, SHI Y, et al. Mutation of glycosylation sites in BST-2 leads to its accumulation at intracellular CD63-positive vesicles without affecting its antiviral activity against multivesicular body-targeted HIV-1 and hepatitis B virus[J]. Viruses, 2016, 8(3): 62. DOI:10.3390/v8030062 |
| [11] | FU Y X, ZHANG L, ZHANG F, et al. Exosome-mediated miR-146a transfer suppresses type I interferon response and facilitates EV71 infection[J]. PLoS Pathog, 2017, 13(9): e1006611. DOI:10.1371/journal.ppat.1006611 |
| [12] | MAHAUAD-FERNANDEZ W D, DEMALI K A, OLIVIER A K, et al. Bone marrow stromal antigen 2 expressed in cancer cells promotes mammary tumor growth and metastasis[J]. Breast Cancer Res, 2014, 16(6): 493. DOI:10.1186/s13058-014-0493-8 |
| [13] | WANG W, NISHIOKA Y, OZAKI S, et al. HM1.24(CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody[J]. Cancer Immunol Immunother, 2009, 58(6): 967–976. DOI:10.1007/s00262-008-0612-4 |
| [14] | FANG K H, KAO H K, CHI L M, et al. Overexpression of BST2 is associated with nodal metastasis and poorer prognosis in oral cavity cancer[J]. Laryngoscope, 2014, 124(9): E354–E360. DOI:10.1002/lary.24700 |
| [15] | BLASIUS A L, GIURISATO E, CELLA M, et al. Bone marrow stromal cell antigen 2 is a specific marker of type Ⅰ IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation[J]. J Immunol, 2006, 177(5): 3260–3265. DOI:10.4049/jimmunol.177.5.3260 |
| [16] | HINZ A, MIGUET N, NATRAJAN G, et al. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain[J]. Cell Host Microbe, 2010, 7(4): 314–323. DOI:10.1016/j.chom.2010.03.005 |
| [17] | SCHUBERT H. L, ZHAI Q T, SANDRIN V, et al. Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations[J]. Proc Natl Acad Sci U S A, 2010, 107(42): 17951–17956. DOI:10.1073/pnas.1008206107 |
| [18] | SWIECKI M, SCHEAFFER S M, ALLAIRE M, et al. Structural and biophysical analysis of BST-2/tetherin ectodomains reveals an evolutionary conserved design to inhibit virus release[J]. J Biol Chem, 2011, 286(4): 2987–2997. DOI:10.1074/jbc.M110.190538 |
| [19] | ROLLASON R, KOROLCHUK V, HAMILTON C, et al. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif[J]. J Cell Sci, 2007, 120(Pt 21): 3850–3858. |
| [20] | MATSUDA A, SUZUKI Y, HONDA G, et al. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways[J]. Oncogene, 2003, 22(21): 3307–3318. DOI:10.1038/sj.onc.1206406 |
| [21] | DUFRASNE F. E, LUCCHETTI M, MARTIN A, et al. Modulation of the NF-κB signaling pathway by the HIV-2 envelope glycoprotein and its incomplete BST-2 antagonism[J]. Virology, 2018, 513: 11–16. DOI:10.1016/j.virol.2017.09.024 |
| [22] | MUKAI S, OUE N, OSHIMA T, et al. Overexpression of transmembrane protein BST2 is associated with poor survival of patients with esophageal, gastric, or colorectal cancer[J]. Ann Surg Oncol, 2017, 24(2): 594–602. DOI:10.1245/s10434-016-5100-z |
| [23] | SAYEED A, LUCIANI-TORRES G, MENG Z, et al. Aberrant regulation of the BST2(Tetherin) promoter enhances cell proliferation and apoptosis evasion in high grade breast cancer cells[J]. PLoS One, 2013, 8(6): e67191. DOI:10.1371/journal.pone.0067191 |
| [24] | MAHAUAD-FERNANDEZ W D, OKEOMA C M. Cysteine-linked dimerization of BST-2 confers anoikis resistance to breast cancer cells by negating proapoptotic activities to promote tumor cell survival and growth[J]. Cell Death Dis, 2017, 8(3): e2687. DOI:10.1038/cddis.2017.68 |
| [25] | NAUSHAD W, MAHAUAD-FERNANDEZ W D, OKEOMA C M. Structural determinant of BST-2-mediated regulation of breast cancer cell motility:A role for cytoplasmic tail tyrosine residues[J]. Oncotarget, 2017, 8(66): 110221–110233. |
| [26] | THAM T. Human papillomavirus and world health organization type Ⅲ nasopharyngeal carcinoma:Multicenter study from an endemic area in Southern China[J]. Cancer, 2019, 125(1): 161. |
| [27] | CHIANG S F, KAN C Y, HSIAO Y C, et al. Bone marrow stromal antigen 2 is a novel plasma biomarker and prognosticator for colorectal carcinoma:A secretome-based verification study[J]. Dis Markers, 2015, 2015: 874054. |
| [28] | PEGTEL D M, SUBRAMANIAN A, MERITT D, et al. IFN-alpha-stimulated genes and Epstein-Barr virus gene expression distinguish WHO type Ⅱ and Ⅲ nasopharyngeal carcinomas[J]. Cancer Res, 2007, 67(2): 474–481. DOI:10.1158/0008-5472.CAN-06-1882 |
| [29] | MERCIECA S, BELDERBOS J S A, VAN BAARDWIJK A, et al. The impact of training and professional collaboration on the interobserver variation of lung cancer delineations:a multi-institutional study[J]. Acta Oncol, 2019, 58(2): 200–208. DOI:10.1080/0284186X.2018.1529422 |
| [30] | YU H L, WANG Y H, WANG S S, et al. Paclitaxel-loaded core-shell magnetic nanoparticles and cold atmospheric plasma inhibit non-small cell lung cancer growth[J]. ACS Appl Mater Interfaces, 2018, 10(50): 43462–43471. DOI:10.1021/acsami.8b16487 |
| [31] | BECKER M, SOMMER A, KRÁTZSCHMAR J R, et al. Distinct gene expression patterns in a Tamoxifen-sensitive human mammary carcinoma xenograft and its Tamoxifen-resistant subline MaCa 3366/TAM[J]. Mol Cancer Ther, 2005, 4(1): 151–168. |
| [32] | HOTTER D, SAUTER D, KIRCHHOFF F. Emerging role of the host restriction factor tetherin in viral immune sensing[J]. J Mol Biol, 2013, 425(24): 4956–4964. DOI:10.1016/j.jmb.2013.09.029 |
| [33] | IKUSHIMA H, MIYAZONO K. TGFbeta signalling:a complex web in cancer progression[J]. Nat Rev Cancer, 2010, 10(6): 415–424. DOI:10.1038/nrc2853 |
| [34] | MAHAUAD-FERNANDEZ W D, BORCHERDING N C, ZHANG W Z, et al. Bone marrow stromal antigen 2(BST-2) DNA is demethylated in breast tumors and breast cancer cells[J]. PLoS One, 2015, 10(4): e0123931. DOI:10.1371/journal.pone.0123931 |
| [35] | WANG J C, BIAN S, LIU M C, et al. Cloning, identification, and functional analysis of bone marrow stromal cell antigen-2 from sika deer (Cervus nippon)[J]. Gene, 2018, 661: 133–138. DOI:10.1016/j.gene.2018.03.101 |
| [36] | WU Y S, LEE Z Y, CHUAH L H, et al. Epigenetics in metastatic breast cancer:its regulation and implications in diagnosis, prognosis and therapeutics[J]. Curr Cancer Drug Targets, 2019, 19(2): 82–100. DOI:10.2174/1568009618666180430130248 |
| [37] | ROMAGNOLO D F, DANIELS K D, Grunwald J T, et al. Epigenetics of breast cancer:Modifying role of environmental and bioactive food compounds[J]. Mol Nutr Food Res, 2016, 60(6): 1310–1329. DOI:10.1002/mnfr.201501063 |
| [38] | HERMAN J G, BAYLIN S B. Gene silencing in cancer in association with promoter hypermethylation[J]. N Engl J Med, 2003, 349(21): 2042–2054. DOI:10.1056/NEJMra023075 |
| [39] | EHRLICH M. DNA hypomethylation, cancer, the immunodeficiency, centromeric region instability, facial anomalies syndrome and chromosomal rearrangements[J]. J Nutr, 2002, 132(8 Suppl): 2424S–2429S. |
| [40] | MAHAUAD-FERNANDEZ W D, OKEOMA C M. B49, a BST-2-based peptide, inhibits adhesion and growth of breast cancer cells[J]. Sci Rep, 2018, 8(1): 4305. DOI:10.1038/s41598-018-22364-z |
| [41] | SAYEED A, MENG Z, LUCIANI G, et al. Negative regulation of UCP2 by TGFβ signaling characterizes low and intermediate-grade primary breast cancer[J]. Cell Death Dis, 2010, 1: e53. DOI:10.1038/cddis.2010.30 |
| [42] | WANG D, KANUMA T, MIZUNUMA H, et al. Analysis of specific gene mutations in the transforming growth factor-beta signal transduction pathway in human ovarian cancer[J]. Cancer Res, 2000, 60(16): 4507–4512. |
| [43] | WEI D Y, LE X D, ZHENG L Z, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis[J]. Oncogene, 2003, 22(3): 319–329. DOI:10.1038/sj.onc.1206122 |
| [44] | XIE T X, HUANG F J, ALDAPE K D, et al. Activation of stat3 in human melanoma promotes brain metastasis[J]. Cancer Res, 2006, 66(6): 3188–3196. DOI:10.1158/0008-5472.CAN-05-2674 |
| [45] | CAI D Q, CAO J, LI Z, et al. Up-regulation of bone marrow stromal protein 2(BST2) in breast cancer with bone metastasis[J]. BMC Cancer, 2009, 9: 102. DOI:10.1186/1471-2407-9-102 |
| [46] | GALÃO R P, LE TORTOREC A, PICKERING S, et al. Innate sensing of HIV-1 assembly by Tetherin induces NFκB-dependent proinflammatory responses[J]. Cell Host Microbe, 2012, 12(5): 633–644. DOI:10.1016/j.chom.2012.10.007 |
 2019, Vol. 45
2019, Vol. 45


