扩展功能
文章信息
- 许东亮, 彭朝晖, 熊美才
- XU Dongliang, PENG Zhaohui, XIONG Meicai
- 姜黄素对骨质疏松大鼠种植体骨结合的促进作用
- Promotion effect of curcumin on implant osseointegration in osteoporosis rats
- 吉林大学学报(医学版), 2019, 45(04): 877-881
- Journal of Jilin University (Medicine Edition), 2019, 45(04): 877-881
- 10.13481/j.1671-587x.20190423
-
文章历史
- 收稿日期: 2018-09-26
2. 平煤集团总医院口腔科, 河南 郑州 450003;
3. 河南省郏县人民医院口腔科, 河南 郏县 467100
2. Department of Stomatology, General Hospital of Pingmei Group, Zhengzhou 450003, China;
3. Department of Stomatology, Jia County People's Hospital, Jia County 467100, China
骨质疏松患者的特征为全身骨量减少,骨组织破坏,从而引起骨脆性增加,骨折发生率升高。目前治疗各种牙齿缺失的主要方法为种植修复,种植手术经常面临的问题为种植区骨量不足,骨质疏松是牙种植失败的主要危险因素之一,可降低种植体骨整合的质量,是口腔种植的相对禁忌证,因此抑制骨吸收、改善骨代谢和促进骨质疏松种植体骨结合可有效提高种植体成功率[1-4]。姜黄素具有多种药理活性[5],对骨质疏松具有预防作用[6],但姜黄素对骨质疏松种植体骨结合的影响尚不清楚。本文作者通过建立去卵巢骨质疏松大鼠模型,并植入种植体,探讨姜黄素对骨质疏松大鼠种植体骨结合的影响,为其临床应用提供依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器健康、清洁级SD大鼠45只,均为雌性,4月龄、体质量280~320g,购自马鞍山丰原制药有限公司,动物许可证号:SYXK(皖)2016-002。姜黄素(大连美仑生物技术有限公司)。螺纹柱状光滑纯钛螺钉(2mm×5mm)购自西安中邦钛制品有限公司,HP-10扭矩测试仪(广东质信电子有限公司),Y.Cheetah型显微CT机(德国Y-XLON公司)等。
1.2 实验动物分组和模型制备将45只大鼠分为对照组、模型组和姜黄素组,每组15只。模型组和姜黄素组大鼠建立骨质疏松模型:将大鼠麻醉成功后,在无菌条件下分别在大鼠脊柱两侧做长1 cm切口,进入腹腔,切除双侧卵巢;对照组大鼠只切除卵巢旁少量脂肪组织,不切除卵巢。术后均给予青霉素肌注预防感染。卵巢切除3个月时,3组大鼠均在麻醉成功后于双侧股骨远中干骺端植入螺纹钉1枚,术后给予青霉素肌注预防感染。姜黄素组大鼠植入螺纹钉后每天给予姜黄素(110 mg·kg-1)灌胃;对照组和模型组大鼠给予等量生理盐水灌胃,共2个月。
1.3 取材用药结束后处死各组大鼠,取各组大鼠双侧包含种植体的股骨远中段骨组织,各组大鼠右侧骨组织标本用于显微CT检查和骨组织形态学检测,左侧股组织用于生物力学扭矩测试。
1.4 各组大鼠骨组织显微CT检查显微CT参数设定:工作电流43μA,工作电压90 kV,分辨率0.008μm,整合时间0.5s。获得各大鼠骨组织全部图像后,选择种植体周围600 μm范围为感兴趣区域,分析感兴趣区域内骨组织,得到相对骨体积分数(bone volume/tissue volume,BV/TV,单位%)、平均骨小梁粗度(trabecular thickness,Tb.Th,单位mm)、骨小梁间隙(trabecular space,Tb.Sp,单位mm)、平均骨小梁数量(bone trabecular number,Tb.N,单位mm-1)和骨结合指数(osseointegration index,OI,单位%)。
1.5 各组大鼠骨组织形态表现检查显微CT检查结束后,将带种植体的骨组织标本进行非脱钙骨硬组织切片,并进行亚甲基蓝-碱性品红染色,染色后常规封片,显微镜下观察拍照。采用Image-pro Plus软件进行处理:标出种植体外形轮廓线,测量总长度和有骨结合部分的长度,计算得到种植体骨结合率;标出骨组织中种植体2个螺纹最外点,并以该2点连线长度为边长做一正方形,计算得到松质骨区骨量:该区域内矿化骨基质面积占区域总面积的百分比。
1.6 各组大鼠种植体扭矩测定将含种植体的骨组织标本长度修整为15 mm,卡入扭矩测试仪,将扭力扳手固定在头部外露部分,并沿着逆时针方向缓慢用力,记录扭矩范围和种植体松动脱位前的最大载荷值,得到脱位扭矩(单位N·cm)。
1.7 统计学分析采用SPSS 20.0统计软件进行统计学分析。BV/TV、Tb.Sp、Tb.Th、Tb.N、OI、骨结合率、松质骨区骨量和脱位扭矩以x±s表示,采用Kolmogorov-Smirnov检验分析数据是否符合正态分布,3组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠一般情况实验过程中3组大鼠均状况良好,无死亡,卵巢切除和种植体植入术后伤口均无感染,愈合良好。
2.2 各组大鼠术后3个月种植体周围骨界面显微CT扫描结果各组大鼠种植体周围骨界面BV/TV、Tb.Sp、Tb.Th、Tb.N和OI比较差异均有统计学意义(P < 0.01)。与对照组比较,模型组和姜黄素组大鼠种植体周围骨界面BV/TV、Tb.Th、Tb.N和OI明显降低(P < 0.01),Tb.Sp明显升高(P < 0.01);与模型组比较,姜黄素组大鼠种植体周围骨界面BV/TV、Tb.Th、Tb.N和OI明显升高(P < 0.01),Tb.Sp明显降低(P < 0.01)。见表 1。
| (n=15, x±s) | |||||
| Group | BV/TV(η/%) | Tb.Sp(l/mm) | Tb.Th(l/mm) | Tb.N(mm-1) | OI(η/%) |
| Control | 78.54±5.12 | 0.11±0.02 | 0.24±0.04 | 8.04±0.38 | 78.42±3.74 |
| Model | 19.09±3.01* | 0.48±0.06* | 0.11±0.01* | 3.42±0.25* | 22.13±1.43* |
| Curcumin | 54.32±4.75*△ | 0.25±0.02*△ | 0.17±0.02*△ | 6.51±0.32*△ | 51.24±2.31*△ |
| F | 695.324 | 356.932 | 103.136 | 805.854 | 1668.818 |
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| *P < 0.01 compared with control group;△P < 0.01 compared with model group. | |||||
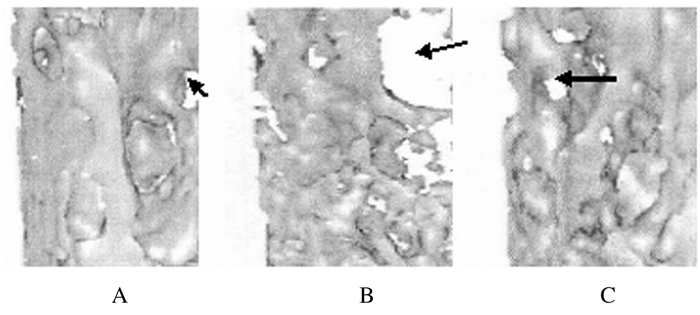
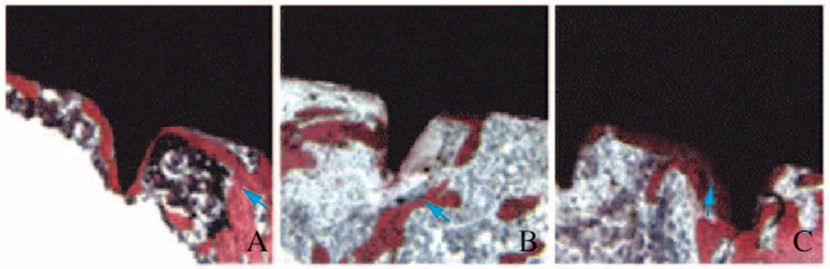
对照组大鼠种植体和骨结合紧密,种植体骨结合界面骨板较厚,骨小梁间隙较小且密集;模型组大鼠种植体骨结合界面骨板比较薄,骨小梁间隙大且较稀疏;姜黄素组大鼠骨组织形态表现介于对照组和模型组之间,种植体骨结合界面骨板较厚,骨小梁间隙较小且密集。见图 1和图 2(插页七)。
|
| Note:Black arrow referred to trabecular space.A: Control group; B: Modelgroup; C: Curcumin group 图 1 各组大鼠种植体骨结合界面显微CT检查结果(×400) Fig. 1 Micro-CT examination results of implant and bone bondinginterface ofrats in various groups(×400) |
|
|
|
| A: Control group; B: Model group; C: Curcumin group.Blue arrow referred to the connecting bone plate. 图 2 各组大鼠种植体骨组织结合界面亚甲基蓝-碱性品红染色结果(×400) Fig. 2 Results of methylene blue-basic fuchsin staining of bone tissue bonding interface of implants of rats in various groups (×400) |
|
|
各组大鼠骨结合率和松质骨区骨量比较差异均有统计学意义(P < 0.01)。与对照组比较,模型组和姜黄素组大鼠骨结合率和松质骨区骨量明显降低(P < 0.01);与模型组比较,姜黄素组大鼠骨结合率和松质骨区骨量明显升高(P < 0.01)。见表 2。
| (n=15, x±s, η/%) | ||
| Group | Bone contact rate | Bone mass in cancellous bone area |
| Control | 77.32±3.52 | 37.13±3.24 |
| Model | 42.15±2.58* | 18.27±2.56* |
| Curcumin | 65.35±3.24*△ | 29.85±3.18*△ |
| F | 487.008 | 149.868 |
| P | < 0.01 | < 0.01 |
| *P < 0.01 compared with control group;△P < 0.01 compared with model group. | ||
各组大鼠种植体脱位扭矩比较差异均有统计学意义(P < 0.01)。与对照组比较,模型组和姜黄素组大鼠种植体脱位扭矩明显降低(P < 0.01);与模型组比较,姜黄素组大鼠种植体脱位扭矩明显升高(P < 0.01)。见表 3。
| (n=15, N·cm) | ||
| Group | Dislocation torque | Torque range |
| Control | 22.01±3.04 | 18.78-24.35 |
| Model | 11.73±2.31* | 9.32-14.59 |
| Curcumin | 18.46±2.75*△ | 16.57-21.46 |
| F | 55.411 | |
| P | < 0.01 | |
| *P < 0.01 compared with control group;△P < 0.01 compared with model group. | ||
随着人口的老龄化,牙齿缺失的发生率不断增加,致使越来越多的骨质疏松老年患者寻求种植牙修复缺失牙齿,但骨质疏松患者骨量减少、脆性增加和微结构破坏,使其种植牙的失败率比较高,从而使大量骨质疏松牙缺失患者的需求不能得以满足[7-8]。骨质疏松对口腔种植的影响主要表现在种植体周围骨质量差、骨量少和种植体骨结合率低[9-11]。雌激素和催产素等疗法可促进骨质疏松动物种植体骨结合。研究[12]显示:骨质疏松大鼠给予催产素治疗,可增加种植体周围BV/TV、OI和最大推出力等,从而拮抗骨质疏松对种植体的负面影响,促进种植体骨结合;王买全等[13]对去卵巢骨质疏松大鼠给予全身应用雌激素和局部应用二膦酸盐治疗,结果显示:雌激素和二膦酸盐治疗也可增加种植体周围BV/TV、OI、最大推出力和固位力等,从而促进种植体骨结合。虽然催产素和雌激素等对促进骨质疏松大鼠种植体骨结合具有较好效果,但两者对子宫、乳腺等靶器官具有一定不良影响,因此寻找无明显不良反应的药物具有重要意义。
姜黄素为一种姜黄素植物根茎中的活性成分,溶于乙醇,不溶于水和乙醚,具有抗癌、抗氧化、抗炎、抗结核和神经保护等多种药理学活性[14-16];在骨骼系统中,姜黄素可抑制破骨细胞增生,也可抑制成骨细胞增生,对骨质疏松也有预防作用[17];姜黄素通过在细胞水平调控多种靶分子参与骨重塑的调控,恢复破骨细胞介导的骨吸收和成骨细胞介导的骨形成之间的平衡关系,抑制骨质丢失,从而预防骨质疏松的发生[18-19]。李义等[20]发现:姜黄素可通过调控骨保护素/核因子κB受体活化因子配基系统扭转去卵巢骨质疏松大鼠骨形态学和血清学指标的变化,发挥抗骨质疏松作用。研究[21]显示:姜黄素通过抑制组织蛋白酶K的表达提高高脂诱导的骨质疏松小鼠模型的骨强度、改善骨微结构,从而发挥对骨质疏松的骨保护作用。鉴于姜黄素具有抗骨质疏松的作用,本文作者发现:与正常对照组比较,模型组大鼠种植体骨结合界面骨板比较薄,骨小梁稀疏,种植体周围骨界面BV/TV、Tb.Th、Tb.N、OI、骨结合率、松质骨区骨量和脱位扭矩降低,Tb.Sp升高;给予姜黄素治疗后,骨质疏松大鼠种植体骨结合界面骨板和骨小梁较密集,种植体周围骨界面BV/TV、Tb.Th、Tb.N、OI、骨结合率、松质骨区骨量和脱位扭矩升高,Tb.Sp降低。本研究结果表明:姜黄素可增加骨质疏松大鼠种植体骨结合的强度和稳定性,其机制可能为:姜黄素具有抗骨质疏松作用,通过对骨重塑的调控抑制骨质丢失,从而增加种植体周围骨量,增加种植体骨结合的强度和稳定性。
综上所述,姜黄素可改善骨质疏松大鼠种植体周围骨质量,增加骨结合强度和稳定性,但姜黄素对种植体骨结合影响的具体机制尚有待进一步深入研究。
| [1] | CAI J, LI W, SUN T, et al. Pulsed electromagnetic fields preserve bone architecture and mechanical properties and stimulate porous implant osseointegration by promoting bone anabolism in type 1 diabetic rabbits[J]. Osteoporos Int, 2018, 29(5): 1177–1191. DOI:10.1007/s00198-018-4392-1 |
| [2] | DUAN Y, MA W, LI D, et al. Enhanced osseointegration of titanium implants in a rat model of osteoporosis using multilayer bone mesenchymal stem cell sheets[J]. Exp Ther Med, 2017, 14(6): 5717–5726. |
| [3] | ZHAO R, XIE PF, ZHANG K, et al. Selective effect of hydroxyapatite nanoparticles on osteoporotic and healthy bone formation correlates with intracellular calcium homeostasis regulation[J]. Acta Biomater, 2017, 59: 338–350. DOI:10.1016/j.actbio.2017.07.009 |
| [4] | CHOI Y H, HAN Y, JIN S W, et al. Pseudoshikonin Ⅰ enhances osteoblast differentiation by stimulating Runx2 and Osterix[J]. J Cell Biochem, 2018, 119(1): 748–757. |
| [5] | PEDDADA K V, PEDDADA K V, SHUKLA S K, et al. Role of curcumin in common musculoskeletal disorders:a review of current laboratory, translational, and clinical data[J]. Orthop Surg, 2015, 7(3): 222–231. DOI:10.1111/os.12183 |
| [6] | XIN M, YANG Y, ZHANG D, et al. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamine D receptor expression[J]. Osteoporos Int, 2015, 26(11): 2665–2676. DOI:10.1007/s00198-015-3153-7 |
| [7] | APOSTU D, LUCACIU O, BERCE C, et al. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty:a review[J]. J Int Med Res, 2018, 46(6): 2104–2119. DOI:10.1177/0300060517732697 |
| [8] | OKI Y, DOI K, MAKIHARA Y, et al. Effects of continual intermittent administration of parathyroid hormone on implant stability in the presence of osteoporosis:An in vivo study using resonance frequency analysis in a rabbit model[J]. J Appl Oral Sci, 2017, 25(5): 498–505. DOI:10.1590/1678-7757-2016-0561 |
| [9] | GHANEM A, KELLESARIAN S V, ABDULJABBAR T, et al. Role of osteogenic coatings on implant surfaces in promoting bone-to-implant contact in experimental osteoporosis:A systematic review and meta-analysis[J]. Implant Dent, 2017, 26(5): 770–777. DOI:10.1097/ID.0000000000000634 |
| [10] | LIU YM, HU J, LIU B, et al. The effect of osteoprotegerin on implant osseointegration in ovariectomized rats[J]. Arch Med Sci, 2017, 13(2): 489–495. |
| [11] | ZHOU HB, HOU YF, ZHU ZM, et al. Effects of low-intensity pulsed ultrasound on implant osseointegration in ovariectomized rats[J]. J Ultrasound Med, 2016, 35(4): 747–754. DOI:10.7863/ultra.15.01083 |
| [12] | HUANG YM, LIN Y, WU YS, et al. Molecular mechanisms of the inhibitory effects of jiangu granule-containing serum on RANKL-induced osteoclastogenesis[J]. Mol Med Rep, 2017, 16(6): 8420–8426. DOI:10.3892/mmr.2017.7645 |
| [13] | 王买全, 李运峰. 同时应用雌激素和二膦酸盐对骨质疏松大鼠种植体骨结合的累加效应[J]. 实用口腔医学杂志, 2016, 32(5): 640–644. DOI:10.3969/j.issn.1001-3733.2016.05.010 |
| [14] | JEBAHI S, SAOUDI M, FARHAT L, et al. Effect of novel curcumin-encapsulated chitosan-bioglass drug on bone and skin repair after gamma radiation:experimental study on a Wistar rat model[J]. Cell Biochem Funct, 2015, 33(3): 150–159. DOI:10.1002/cbf.3098 |
| [15] | RIVA A, TOGNI S, GIACOMELLI L, et al. Effects of a curcumin-based supplementation in asymptomatic subjects with low bone density:A preliminary 24-week supplement study[J]. Eur Rev Med Pharmacol Sci, 2017, 21(7): 1684–1689. |
| [16] | 李刚, 刘莹, 种铁, 等. 姜黄素对裸鼠人ACHN肾癌移植瘤的放射增敏作用[J]. 西安交通大学学报:医学版, 2019, 40(2): 284–289. |
| [17] | 陈之光, 薛今琦, 付勤, 等. 姜黄素对骨骼系统的影响[J]. 中华骨质疏松和骨矿盐疾病杂志, 2016, 9(3): 323–329. DOI:10.3969/j.issn.1674-2591.2016.03.017 |
| [18] | CHEN Z, XUE J, SHEN T, et al. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway[J]. Int J Mol Med, 2016, 37(2): 329–338. DOI:10.3892/ijmm.2015.2432 |
| [19] | CHO D C, RYU K, KIM K T, et al. The therapeutic effects of combination therapy with curcumin and alendronate on spine fusion surgery in the ovariectomized rats[J]. Korean J Spine, 2017, 14(2): 35–40. DOI:10.14245/kjs.2017.14.2.35 |
| [20] | 李义, 张建中, 宋英, 等. 姜黄素对去卵巢骨质疏松模型鼠的机制研究[J]. 中成药, 2013, 35(7): 1359–1362. DOI:10.3969/j.issn.1001-1528.2013.07.001 |
| [21] | 马如风, 王丽丽, 左加成, 等. 姜黄素通过调节组织蛋白酶K改善高脂诱导C57BL/6J小鼠骨结构和骨质量的实验研究[J]. 中国药理学通报, 2017, 33(10): 1446–1451. DOI:10.3969/j.issn.1001-1978.2017.10.023 |
 2019, Vol. 45
2019, Vol. 45


