扩展功能
文章信息
- 黑利娟, 王娟红, 魏威, 方航荣, 段瑛, 马士芬, 徐俊荣
- HEI Lijuan, WANG Juanhong, WEI Wei, FANG Hangrong, DUAN Ying, MA Shifen, XU Junrong
- 乳头状甲状腺癌患者血清和甲状腺组织中白细胞介素6水平的检测及其意义
- Detection of interleukin-6 levels in serum and thyroid tissue of patients with papillary thyroid carcinoma and their significances
- 吉林大学学报(医学版), 2019, 45(03): 601-605
- Journal of Jilin University (Medicine Edition), 2019, 45(03): 601-605
- 10.13481/j.1671-587x.20190322
-
文章历史
- 收稿日期: 2018-08-28
2. 延安大学医学院, 陕西 延安 716000;
3. 西安交通大学医学院附属西安市中心医院病理科, 陕西 西安 710000;
4. 陕西省西安市第三医院病理科, 陕西 西安 710003
2. College of Medical Sciences, Yan'an University, Yan'an 716000, China;
3. Department of Pathology, Xi'an Central Hospital, School of Medical Sciences, Xi'an Jiaotong University, Xi'an 710003, China;
4. Department of Pathology, Xi'an Third Hospital, Shannxi Province, Xi'an 710000, China
乳头状甲状腺癌(papillary thyroid carcinoma, PTC)是最常见的内分泌系统恶性肿瘤,好发于中、青年女性。大部分PTC患者预后良好,但是部分患者仍存在复发、甚至死亡的危险。近年来,PTC发病率在全世界范围内呈升高趋势[1-4],但其具体发病机制尚不明确。研究[5-6]显示:白细胞介素6(interleukin-6, IL-6)与许多恶性肿瘤的发生发展有密切关系,IL-6及其相关信号通路可能通过促进细胞增殖、迁移和侵袭[7],导致恶性肿瘤患者的不良预后[8],但IL-6与PTC关系的研究尚未见相关报道。有研究者[9]发现:与健康对照组比较,甲状腺疾病组患者血清IL-6水平明显升高。CHUNG等[10]的研究显示:细胞因子IL-18和IL-18受体与PTC的进展和淋巴结转移有关联。因此,本文作者采用ELISA和免疫组织化学法定性及定量检测PTC、桥本甲状腺炎(Hashimoto’ s thyroiditis, HT)并发PTC患者的血清IL-6水平及病变甲状腺组织中IL-6蛋白表达情况,旨在探讨IL-6与PTC的相关性及其可能的作用机制,为PTC的诊治提供依据。
1 资料与方法 1.1 样本和病例来源5例PTC和5例HT并发PTC患者均为2013-2014年陕西省西安市中心医院外科及内分泌科经临床、甲状腺功能检测、手术及病理确诊的病例,分别作为病例1组和病例2组,5名查体正常者为正常组。20例PTC、15例HT并发PTC石蜡组织标本来自2010-2014年陕西省西安市中心医院病理科存档蜡块,分别作为PTC组和HT并发PTC组;同期18例甲状腺腺瘤旁正常甲状腺石蜡组织标本作为对照组,对照组样本取自18例××患者,女性17例,男性1例,年龄25~67岁,平均年龄(46.5±2.6)岁。
1.2 纳入和排除标准纳入标准:患者结合临床症状和体征,均经病理学确诊为PTC或HT并发PTC,未接受过放、化疗或生物治疗等干预措施,均具有完整的病历资料,并签署知情同意书。排除标准:排除存在其他恶性肿瘤、严重心脏和肝肾疾病及严重并发症等情况患者。
1.3 主要试剂和仪器细胞因子IL-6检测ELISA试剂盒购自上海森雄公司,免疫组织化学抗体小鼠抗人IL-6、IL-6受体(IL-6R)、P53和KI-67单克隆抗体及SP二抗试剂盒和DAB显色试剂盒购自福州迈新公司,鼠抗人RET/PTC癌基因单抗和鼠抗人核因子κB(nuclear factor kappa-B,NF-κB) p65单抗购自美国Santa Cruz公司。石蜡组织切片机(型号2135,购自德国徕卡公司),普通光学显微镜(台湾奥林巴斯公司)。
1.4 ELISA法检测研究对象血清IL-6水平各组研究对象(病例1组、病例2组和正常组)均常规采静脉血2 mL,室温静置1 h,3 000 r·min-1分钟离心10 min,取血清1 mL,-70℃保存。采用双抗体夹心ELISA法检测以上研究对象血清中细胞因子IL-6水平。采用全自动酶标分析仪检测450 nm处的吸光度(A)值,依标准品值制作标准曲线,计算细胞因子水平。具体步骤按说明书严格操作。
1.5 免疫组织化学法检测研究对象甲状腺组织中IL-6、IL-6R、RET/PTC和NF-κB蛋白阳性表达率采用免疫组织化学(IHC)法检测研究对象(PTC组、HT并发PTC组和对照组)甲状腺组织中IL-6、IL-6R、NF-κB和RET/PTC蛋白表达,IHC染色采用常规链霉菌素亲生物素-过氧化酶连接法(SP法)。IL-6单抗1:200稀释,IL-6R单抗1:100稀释,RET/PTC单抗1:100稀释,以胞浆呈棕黄色为阳性,NF-κB p65单抗1:400稀释,以胞浆/核呈棕黄色为阳性。阴性对照采用PBS代替一抗或用含有小鼠单抗IgG1的神经胶质酸性蛋白稀释至与一抗相同IgG1浓度代替单抗。
1.6 统计学分析采用SPSS20.0统计软件进行统计学分析。各组研究对象血清IL-6和IL-6R水平以x±s表示,组间比较采用独立样本t检验;研究对象甲状腺组织中IL-6、IL-6R、RET/PTC和NF-κB蛋白阳性表达率组间比较采用χ2检验或Fisher精确检验。IL-6、IL-6R和NF-κB和RET/PTC表达的相关性分析采用配对χ2检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组研究对象甲状腺组织形态表现病例1组PTC患者甲状腺组织内上皮细胞复杂乳头状增生伴细胞核毛玻璃样,并见核沟及核内包涵体;病例2组HT并发PTC患者甲状腺组织中除了PTC典型病变外,还可以看见弥漫淋巴组织浸润伴淋巴滤泡形成,增生与萎缩甲状泡上皮共存伴甲状腺上皮嗜酸性变,间质纤维化;正常组研究对象正常甲状腺组织小叶结构存在,滤泡细胞大小一致。
2.2 各组研究对象血清中IL-6水平与正常组(正常人)比较,病例1组和病例2组患者血清IL-6水平明显升高,其中病例1组患者血清IL-6水平为(15.4±7.8) ng·L-1,病例2组为(18.8±2.6) ng·L-1,正常组为(6.5±3.8) ng·L-1。与正常组比较,病例2组患者血清IL-6水平明显升高(P=0.009),病例1组患者血清IL-6水平升高,但差异无统计学意义(P=0.069);与正常组比较,病例1组和病例2组全部患者血清IL-6水平比较差异有统计学意义(P=0.012)。
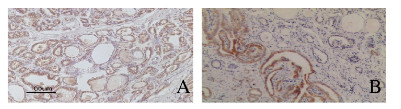
2.3 各组研究对象甲状腺组织中IL-6和IL-6R蛋白阳性表达率与对照组比较,HT并发PTC组和PTC组患者甲状腺组织中IL-6和IL-6R蛋白阳性表达率均明显升高(P < 0.05)。HT并发PTC组可能因为病例数太少,患者甲状腺组织中IL-6和IL-6R蛋白阳性表达率与PTC组比较差异无统计学意义(P>0.05),见表 1。HT并发PTC组和PTC组患者甲状腺组织中IL-6和IL-6R蛋白主要表达于甲状腺病变上皮细胞中,部分表达于浸润的淋巴细胞中,见图 1(插页五)。
| (η/%) | |||
| Group | n | IL-6 | IL-6R |
| Control | 18 | 50.0 | 7.7 |
| PTC | 20 | 100.0* | 46.7* |
| HT+PTC | 15 | 100.0* | 50.0* |
| *P < 0.05 vs control group. | |||
|
| 图 1 PTC患者甲状腺组织中IL-6(A)和IL-6R(B)蛋白的表达(免疫组织化学,×100) Fig. 1 Expressions of IL-6(A) and IL-6R(B) proteins in thyroid tissue of patients with PTC(Immunohistochemistry, ×100) |
|
|
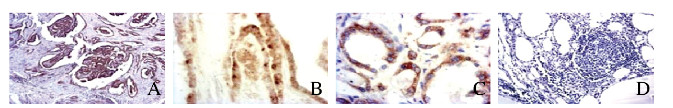
免疫组织化学法检测PTC患者病变甲状腺组织中甲状腺上皮细胞核转录因子NF-κB及PTC发生的特征性基因RET/PTC蛋白的表达结果显示:与对照组比较,HT并发PTC组和PTC组患者病变甲状腺组织中NF-κB P65与RET/PTC蛋白阳性表达率均明显升高(P < 0.05),而且NF-κB p65表达呈活化状态,见表 2及图 2(插页五)。NF-κB与RET/PTC蛋白表达有关联(P < 0.05),IL-6和IL-6R表达均与RET/PTC蛋白表达有关联(P < 0.05)。见表 3。
| (η/%) | |||
| Group | n | NF-κB P65 | RET/PTC |
| Control | 18 | 27.8 | 0 |
| PTC | 20 | 95.0* | 65.0* |
| HT+PTC | 15 | 95.0* | 60.0* |
| *P < 0.05 vs control group. | |||
|
| A-B:PTC tissue; C-D:Normal human thyroid tissue; A:RET/PTC; B-C:NF κB; D:Negative control; A:×40;B:×200;C-D:×100 图 2 免疫组织化学染色检测PTC患者甲状腺组织中RET/PTC和NF-κB蛋白的表达 Fig. 2 Expressions of RET/PTC and NF-κB protein in thyroid tissue of patients with PTC detected by immunohistochemistry |
|
|
| Group | IL-6(η/%) | P | IL-6R(η/%) | P |
| NF-κB | 64.7 | 0.515 | 38.7 | 0.157 |
| RET/PTC | 75.0 | 0.039 | 45.5 | 0.024 |
PTC是源于甲状腺滤泡上皮细胞的恶性肿瘤,为甲状腺癌最常见的病理类型,好发于中、青年女性。近年来,PTC发病率在全世界范围内呈升高趋势[3],且发病年龄年轻化,严重威胁着人类健康,但其具体发病机制尚不明确。PTC临床症状不典型,多数患者于体检时发现,容易错过最佳治疗时间。PTC预后较好,但有部分患者容易复发或在早期出现转移,因此,积极探索PTC发生发展机制,对PTC的早期诊治具有重要意义。
IL-6为巨噬细胞、淋巴细胞、成纤维细胞、内皮细胞和肿瘤细胞等所分泌,是一种重要的多效性细胞因子,几乎参与各个器官的生理活动,并在免疫调节、感染和肿瘤发生等过程中扮演着重要角色。IL-6R广泛表达于多种细胞表面, 其与IL-6结合才能更好地发挥生物学效应。有学者[8]认为IL-6具有促炎和抗炎的双重作用,后来发现IL-6的高表达和异常的信号通路可促进肿瘤的发生,并与许多类型癌症的不良预后有关联。研究[11-12]表明:在肺癌、胃癌和乳腺癌等恶性肿瘤患者血液中IL-6水平明显高于健康对照者。目前认为高水平IL-6能通过调控细胞周期相关基因, 使得细胞异常增殖, 进而促进肿瘤的发生;异常表达的IL-6还可以诱导血管内皮生长因子的合成,促进血管生成和肿瘤生长[13]。由此可见IL-6与恶性肿瘤的发展过程密切相关。
本研究采用ELISA法分别检测HT并发PTC患者和PTC患者血清IL-6水平,结果显示:HT并发PTC组患者血清IL-6水平明显升高。采用IHC法分别检测HT并发PTC患者和PTC患者病变甲状腺组织中IL-6和IR-6R蛋白表达情况,结果显示:与对照组比较,PTC组患者甲状腺病变组织中IL-6和IL-6R蛋白表达水平明显升高,HT并发PCT组患者甲状腺病变组织中IL-6R蛋白表达水平明显升高,提示IL-6可能在PTC发生发展中起重要作用。本研究中IHC法检测结果显示:IL-6和IL-6R蛋白主要表达于甲状腺病变上皮细胞内,部分表达于浸润的淋巴细胞胞浆内,表明肿瘤细胞可能通过自分泌方式产生IL-6,使机体免疫功能受到抑制,实现免疫逃逸,最终促进肿瘤的生长和转移。
炎症与肿瘤之间的关系一直备受关注,有文献[14]报道:慢性炎症是肿瘤发生的基础,并且肿瘤发生过程可能与炎症有类似的介导通路。研究[8, 15]证明:炎症性肠病以及其他慢性炎性疾病与癌症有重要的相关性。越来越多的学者认为HT与PTC关系密切。IL-6在炎症反应和肿瘤形成的过程中扮演重要角色,其是通过激活多种信号通路发挥作用。NF-κB是IL-6/IL-6R的重要信号通路之一,IL-6可通过其促炎因子的作用参与肿瘤的发生发展。本研究结果显示:与对照组比较,PTC组和HT并发PTC组患者病变甲状腺组织中NF-κBp65和RET/PTC蛋白阳性表达率明显升高,且NF-κB表达呈活化状态,IL-6和IL-6R均与RET/PTC蛋白表达有关,提示HT可能为PTC的癌前病变。研究[16]显示:RET/PTC可以通过调节NF-κB促进PTC的发生发展,结合本研究结果,本文作者认为:IL-6在PTC癌变中可能通过参与RET/PTC-NF-κB信号通路发挥作用。
NF-κB是哺乳动物组织中一类重要的转录因子,参与信号转导通路、调节细胞增殖、血管生成和抑制癌细胞凋亡等过程[17]。p65为NF-κB家族重要成员之一。在刺激因素作用下,NF-κBp65被激活,参与白血病、乳腺癌和卵巢癌等多种恶性肿瘤的生长及转移过程[17-18]。研究[19]显示:NF-κB p65过表达同样在甲状腺上皮细胞的恶变过程中起着重要作用。RET/PTC癌基因被认为是PTC发生的特征性基因[20],但近年来在HT非癌性甲状腺滤泡上皮细胞中该基因的检出率也非常高[21-22],提示HT与PTC发展的早期阶段可能存在着相同的分子机制。有文献[16]报道:RET/PTC能通过调节NF-κB活性和相对蛋白表达促进PTC病变细胞的增殖和迁移。活化的NF-κB既能诱导正常上皮细胞发生恶变,还能促进IL-6等多种致炎细胞因子的转录,引起肿瘤发生部位炎细胞浸润,细胞免疫失衡,介导炎症相关性肿瘤的形成和转移。本研究结果显示:IL-6和IL-6R均与RET/PTC蛋白表达有关,IL-6在PTC癌变中可能通过参与RET/PTC-NF-κB信号通路发挥作用。与对照组比较,HT并发PTC和PTC组患者病变甲状腺组织中IL-6和IL-6R蛋白表达水平均明显升高,且IL-6和IL-6R蛋白主要表达于HT和PTC患者病变甲状腺上皮细胞中,这些病变上皮细胞同时表达NF-κB P65和RET/PTC,且IL-6、IL-6R与NF-κB P65、RET/PTC蛋白表达有关联。
综上所述,IL-6在PTC的发生发展中起着重要作用,肿瘤细胞可能通过自分泌方式产生IL-6,促进自身的生长,并且IL-6在PTC癌变中可能通过参与RET/PTC-NF-κB信号通路发挥重要作用。这一结果为PTC的诊断及防治提供了新的理论依据。
| [1] | RAHIB L, SMITH B D, AIZENBERG R, et al. Projecting cancer incidence and deaths to 2030:the unexpected burden of thyroid, liver, and pancreas cancers in the United States[J]. Cancer Res, 2014, 74(11): 2913–2921. DOI:10.1158/0008-5472.CAN-14-0155 |
| [2] |
EROL V, MAKAY Ö, |
| [3] | NIX P, NICOLAIDES A, COATESWORTH A P. Thyroid cancer review 1:presentation and investigation of thyroid cancer[J]. Int J Clin Pract, 2005, 59(11): 1340–1344. DOI:10.1111/ijcp.2005.59.issue-11 |
| [4] | VITA R, IENI A, TUCCARI G, et al. The increasing prevalence of chronic lymphocytic thyroiditis in papillary microcarcinoma[J]. Rev Endocr Metab Disord, 2018, 19(4): 301–309. DOI:10.1007/s11154-018-9474-z |
| [5] | KNÜPFER H, PREISS R. Serum interleukin-6 levels in colorectal cancer patients-a summary of published results[J]. Int J Colorectal Dism, 2010, 25(2): 135–140. DOI:10.1007/s00384-009-0818-8 |
| [6] | 姜春霞, 陈阔. 胃癌患者血清中肿瘤坏死因子-α和白介素-6水平检测及其临床诊断价值[J]. 肿瘤基础与临床, 2017, 30(1): 74–75. DOI:10.3969/j.issn.1673-5412.2017.01.023 |
| [7] | SANTER F R, MALINOWSKA K, CULIG Z, et al. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells[J]. Endocr Relat Cancer, 2010, 17(1): 241–253. DOI:10.1677/ERC-09-0200 |
| [8] | YEH K Y, LI Y Y, HSIEH L L, et al. Analysis of the effect of serum interleukin-6(IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer[J]. Jpn J Clin Oncol, 2010, 40(6): 580–587. DOI:10.1093/jjco/hyq010 |
| [9] | PROVATOPOULOU X, GEORGIADOU D, SERGENTANIS T N, et al. Interleukins as markers of inflammation in malignant and benign thyroid disease[J]. Inflamm Res, 2014, 63(8): 667–674. DOI:10.1007/s00011-014-0739-z |
| [10] | CHUNG J H, LEE Y C, EUN Y G, et al. Single nucleotide polymorphism of interleukin-18 and interleukin-18 receptor and the risk of papillary thyroid cancer[J]. Exp Clin Endocrinol Diabetes, 2015, 123(10): 598–603. DOI:10.1055/s-00000017 |
| [11] | 杨毅, 张洁, 王智勇, 等. SHP2介导IL-6促进乳腺癌细胞侵袭作用机制的研究[J]. 中国肿瘤临床, 2016, 43(18): 792–796. DOI:10.3969/j.issn.1000-8179.2016.18.744 |
| [12] | LUMACHI F, BASSO S M M, ORLANDO R. Cytokines, thyroid diseases and thyroid cancer[J]. Cytokine, 2010, 50(3): 229–233. DOI:10.1016/j.cyto.2010.03.005 |
| [13] | HOLTE E, KLEVELAND O, UELAND T, et al. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infarction[J]. Heart, 2017, 103(19): 1521–1527. DOI:10.1136/heartjnl-2016-310875 |
| [14] | BARTON B E, MURPHY T F. Cancer cachexia is mediated in part by the induction of IL-6-like cytokines from the spleen[J]. Cytokine, 2001, 16(6): 251–257. DOI:10.1006/cyto.2001.0968 |
| [15] | TONG J, WANG Y, DA J P. Usefulness of CK19, HBME-1 and galectin-3 expressions in differential diagnosis of thyroid papillary microcarcinoma from benign lesions[J]. Zhonghua Zhong Liu Za Zhi, 2011, 33(8): 599–604. |
| [16] | ZHOU DH, LI Z, BAI X F. BRAFV600E and RET/PTC promote proliferation and migration of papillary thyroid carcinoma cells in vitro by regulating nuclear factor-κB[J]. Med Sci Monit, 2017, 23: 5321–5329. DOI:10.12659/MSM.904928 |
| [17] | HONG L L, QIN J S, HUANG J X, et al. Expression and significance of NF-κB p65 and CyclinD1 in papillary thyroid carcinoma[J]. Hainan Med J, 2014, 11(12): 1853–1855. |
| [18] | PRASAD S, RAVINDRAN J, AGGARWAL B B. NF-κB and cancer:how intimate is this relationship[J]. Mol Cell Biochem, 2010, 336(1/2): 25–37. |
| [19] | VISCONTI R, CERUTTI J, BATTISTA S, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkappaB p65 protein expression[J]. Oncogene, 1997, 15(16): 1987–1994. DOI:10.1038/sj.onc.1201373 |
| [20] | FAGIN J A. How thyroid tumors start and why it matters:kinase mutants as targets for solid cancer pharmacotherapy[J]. J Endocrinol, 2004, 183(2): 249–256. |
| [21] | RHODEN K J, UNGER K, SALVATORE G, et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes:follicular cells of Hashimoto's thyroiditis share low-level recombination events with a subset of papillary carcinoma[J]. J Clin Endocrinol Metab, 2006, 91(6): 2414–2423. DOI:10.1210/jc.2006-0240 |
| [22] | WIRTSCHAFTER A, SCHMIDT R, ROSEN D, et al. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis[J]. Laryngoscope, 1997, 107(1): 95–100. DOI:10.1097/00005537-199701000-00019 |
 2019, Vol. 45
2019, Vol. 45


