扩展功能
文章信息
- 郭佳, 宋思奇, 王克宇
- GUO Jia, SONG Siqi, WANG Keyu
- 热射病小鼠大脑皮层组织中炎症细胞因子水平及P38MAPK/P65NF-κB信号通路变化
- Changes of inflammatory cytokine levels and P38MAPK/P65NF-κB signaling pathway in cerebral cortex tissue of mice with heat stroke
- 吉林大学学报(医学版), 2019, 45(03): 566-571
- Journal of Jilin University (Medicine Edition), 2019, 45(03): 566-571
- 10.13481/j.1671-587x.20190316
-
文章历史
- 收稿日期: 2018-08-07
2. 华北理工大学冀唐学院图书馆, 河北 唐山 063000
2. Library of JiTang College, North China University of Technology, Tangshan 063000, China
神经细胞在病理因素的刺激下可激活P38丝裂原活化蛋白激酶(P38 mitogen-activated protein kinase,P38MAPK),进而激活MAPK信号通路,分泌白细胞介素1β(interleukin-1β, IL-1β)和肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)等炎性细胞因子,引起神经系统损伤[1],同时神经元内的蛋白激酶降解释放具有活性的核转录因子κB(nuclear transcription factor-κB,NF-κB),后者进入细胞核诱导IL-1β、IL-6和TNF-α等炎性靶基因转录,从而激活脑内炎性细胞,发生正反馈炎症级联反应[2]。热射病是一种重症中暑,是在高温高湿环境中引起核心温度快速升高至40℃以上,伴有惊厥、谵妄和昏迷等中枢神经系统功能障碍的多器官系统损伤的一种严重临床综合征,其病理机制类似于全身脓毒血症的一种全身炎症反应综合征。研究[3]显示:热射病小鼠中枢神经系统存在炎症反应,但其炎症反应是否与P38MAPK/P65NF-κB信号通路的激活有关尚不十分清楚。本文作者对热射病小鼠脑组织炎症细胞因子水平及P38MAPK/P65NF-κB信号通路进行研究,探讨其中枢神经系统炎症反应发生的可能机制。
1 材料与方法 1.1 实验动物健康、清洁级、12周龄、雄性BALA/c小鼠60只, 体质量27~31 g, 购自北京市医疗器械检验所,动物许可证号:SYXK(京)2015-0005。
1.2 主要试剂和仪器特定环境智能型模拟实验舱(HOPE-MED8105E型,天津合普公司),Quantity One4.6.2(Universal HoodⅡ,美国Bio-Rad公司),兔抗P38MAPK抗体、兔抗P65NF-κB抗体、兔抗p-P38MAPK抗体、兔抗p-P65NF-κB抗体和辣根过氧化物酶记山羊抗兔IgG抗体(美国Proteinech Group公司),ECL发光液、BCA法蛋白定量试剂盒和蛋白提取试剂盒(美国Advansta公司),HE染色试剂盒和RIPA裂解液(美国Promega公司),IL-1β、IL-6和TNF-α ELISA试剂盒(美国EBioscience公司)。Olympus BX2生物荧光显微镜(日本奥林巴斯公司),FC型酶标仪和化学发光成像仪(美国Thermo公司)。
1.3 实验动物分组及给药将60只小鼠根据随机数字法分为对照组及热射病1、6和24 h组(即建立热射病模型小鼠出舱后1、6和24 h, 并分别在各组对应时间点进行大脑皮层组织相关指标的检测),每组15只。
热射病小鼠模型的建立:将小鼠置入特定环境智能型模拟实验舱中进行热刺激、设置温度为(41.2±0.5)℃,湿度为(60±2)%,当小鼠肛温达(42.0±0.5)℃时出舱;对照组小鼠置于同样环境模拟舱中,温度为(25.0±0.5)℃、湿度为(60±2)%。
恢复期肛温的监测:热射病1、6和24 h组小鼠出舱后与对照组小鼠共同在温度为(25.0±0.5)℃、湿度为(60±2)%的环境中饲养,并同时恢复饮食饮水。测定造模前后各组小鼠体质量并对比,测定各组小鼠造模前后肛温。
1.4 各组小鼠脑组织取材各组小鼠达相应恢复时间点后,腹腔注射乌巴比(40 mg·kg-1)麻醉,麻醉成功后固定于手术台上,剪开腹腔、膈肌和肋骨,暴露胸腔,分离小鼠心脏周围组织,灌注针插入主动脉口并固定,剪开右心耳,生理盐水灌注至右心耳流出澄清生理盐水提示灌注彻底,随即分离各组小鼠双侧大脑皮层组织,左侧大脑皮层组织一半用于HE染色、一半用于ELISA法检测;右侧大脑皮层组织用于Western blotting法检测。
1.5 各组小鼠大脑皮层组织的HE染色将各组小鼠脑组织于多聚甲醛中固定,石蜡包埋,切成厚4 μm切片,常规进行HE染色,荧光显微镜下观察各组小鼠大脑皮层组织形态表现。
1.6 各组小鼠大脑皮层组织中IL-1β、IL-6和TNF-α水平测定将各组小鼠大脑皮层组织置入装有RIPA裂解液的研磨管中研磨60 s,取上清液,采用ELISA法测定小鼠大脑皮层组织中IL-1β、IL-6和TNF-α水平。
1.7 各组小鼠大脑皮层组织中P38MAPK、P65NF-κB、p-P38MAPK和p-P65NF-κB蛋白表达水平测定将小鼠大脑皮层组织置入含RAPI蛋白裂解液的研磨管中研磨60 s,提取总蛋白质,采用CBA法测定其蛋白浓度,采用Western blotting法测定其蛋白水平,一抗为兔抗4种抗体,以β-actin为内参照,采用Quantity One4.6.2对图像上的条带进行分析,计算蛋白表达水平。蛋白表达水平=蛋白条带灰度值/β-actin条带灰度值。1.8统计学分析采用SPSS20.0统计软件进行统计学分析。小鼠体质量丢失和肛温,大脑皮层组织中IL-1β、IL-6和TNF-α水平,大脑皮层组织中P38MAPK、P65NF-κB、p-P38MAPK和p-P65NF-κB蛋白表达水平以x±s表示,多组间样本比较采用单因素方差分析,组间两两比较采用LSD检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠存活情况24 h内,对照组无小鼠死亡;热射病1 h、6 h和24 h组小鼠分别死亡1、1和2只。
2.2 各组小鼠体质量丢失和肛温与对照组比较,热射病1h、6 h和24 h组小鼠体质量丢失明显增加(P < 0.05);随着热射病时间延长,体质量丢失逐渐减少(P < 0.05)。热射病1 h、6 h和24 h组小鼠入舱后肛温迅速升高,并出现躁动,入舱2 h肛温达(40.00±0.48)℃,肛温达(42.40±0.18)℃时出舱,出舱后1 ~ 2 h肛温降至(28.02± 0.65)℃,随后逐渐恢复至正常值。热射病1 h和6 h组小鼠肛温明显低于对照组(P < 0.05),热射病24 h组小鼠肛温与对照组比较差异无统计学意义(P>0.05)。见表 1。
| (x±s) | |||
| Group | n | Loss of body mass (m/mg) | Anal temperature (θ/℃) |
| Control | 15 | 0.02±0.01 | 36.53±0.57 |
| Heat stroke | |||
| 1 h | 14 | 4.03±0.36* | 28.02±0.65* |
| 6 h | 14 | 3.54±0.27*△ | 29.14±0.71*△ |
| 24 h | 13 | 2.98±0.31*△# | 36.78±0.74△# |
| F | 653.204 | 688.435 | |
| P | < 0.001 | < 0.001 | |
| *P < 0.05 compared with control group; △P < 0.05 compared with heatstroke 1 h group; #P < 0.05 compared with heatstroke 6 h group. | |||
对照组小鼠大脑皮层组织形态正常,血管管壁和脑组织连接紧密,管腔形态正常;热射病1 h组小鼠大量脑细胞核固缩、核染色深,血管被压缩变形、管壁外大量水肿;热射病6 h组小鼠核固缩脑细胞数减少,血管外水肿减轻;热射病24 h组小鼠核固缩脑细胞和血管外水肿少见。见图 1(插页四)。
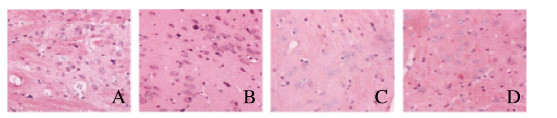
|
| A: Control group; B: Heat strock 1 h group; C: Heat strock 6 h group; D: He at strock 24 h group 图 1 出仓后各组小鼠大脑皮层组织形态表现(HE, ×400) Fig. 1 Morphology of cerebral cortex tissue of mice in various groups after leaving warehouse(HE, ×400) |
|
|
热射病1h和6 h组小鼠大脑皮层组织中IL-1β、IL-6和TNF-α水平明显高于对照组(P < 0.05),热射病6 h组小鼠大脑皮层组织中IL-1β、IL-6和TNF-α水平明显低于热射病1 h组(P < 0.05);热射病24 h组小鼠大脑皮层组织中IL-1β、IL-6和TNF-α水平明显低于热射病1 h和6 h组(P < 0.05),热射病24 h小鼠大脑皮层组织中IL-1β、IL-6和TNF-α水平与对照组比较差异无统计学意义(P>0.05)。见表 2。
| [x±s, wB/(ng·g-1)] | ||||
| Group | n | IL-1β | IL-6 | TNF-α |
| Control | 15 | 4.43±0.37 | 42.54±12.17 | 5.02±0.56 |
| Heat stroke | ||||
| 1 h | 14 | 12.58±0.52* | 172.68±24.35* | 17.58±1.54* |
| 6 h | 14 | 9.41±0.48*△ | 112.84±15.46*△ | 10.73±0.87*△ |
| 24 h | 13 | 4.51±0.42△# | 43.11±11.96△# | 5.21±0.62△# |
| F | 1 096.452 | 195.754 | 514.528 | |
| P | < 0.001 | < 0.001 | < 0.001 | |
| *P < 0.05 compared with control group; △P < 0.05 compared with heat stroke 1 h group; #P < 0.05 compared with heat strock 6 h group. | ||||
各组小鼠大脑皮层组织中P38MAPK和P65NF-κB蛋白表达水平比较差异无统计学意义(P>0.05);热射病1 h和6 h组小鼠大脑皮层组织中p-P38MAPK和p-P65NF-κB蛋白表达水平明显高于对照组(P < 0.05),热射病6 h组小鼠大脑皮层组织中p-P38MAPK和p-P65NF-κB蛋白表达水平明显低于热射病1 h组(P < 0.05);热射病24 h组小鼠大脑皮层组织中p-P38MAPK和p-P65NF-κB蛋白表达水平明显低于热射病1 h和6 h组(P < 0.05),热射病24 h组小鼠大脑皮层组织中p-P38MAPK和p-P65NF-κB蛋白表达水平与对照组比较差异无统计学意义(P>0.05)。见表 3和图 2。
| (x±s) | |||||
| Group | n | P38MAPK | P65NF-κB | p-P38MAPK | p-P65NF-κB |
| Control | 15 | 0.46±0.13 | 0.37±0.11 | 0.34±0.07 | 0.25±0.04 |
| Heat stroke | |||||
| 1 h | 14 | 0.45±0.12 | 0.42±0.14 | 0.73±0.16* | 0.64±0.12* |
| 6 h | 14 | 0.48±0.09 | 0.39±0.13 | 0.52±0.11*△ | 0.41±0.09*△ |
| 24 h | 13 | 0.47±0.10 | 0.41±0.09 | 0.37±0.13△# | 0.27±0.06△# |
| F | 0.187 | 0.496 | 30.585 | 65.904 | |
| P | 0.905 | 0.687 | < 0.001 | < 0.001 | |
| *P < 0.05 compared with control group; △P < 0.05 compared with heat stroke 1 h group; #P < 0.05 compared with heat stroke 6 h group. | |||||
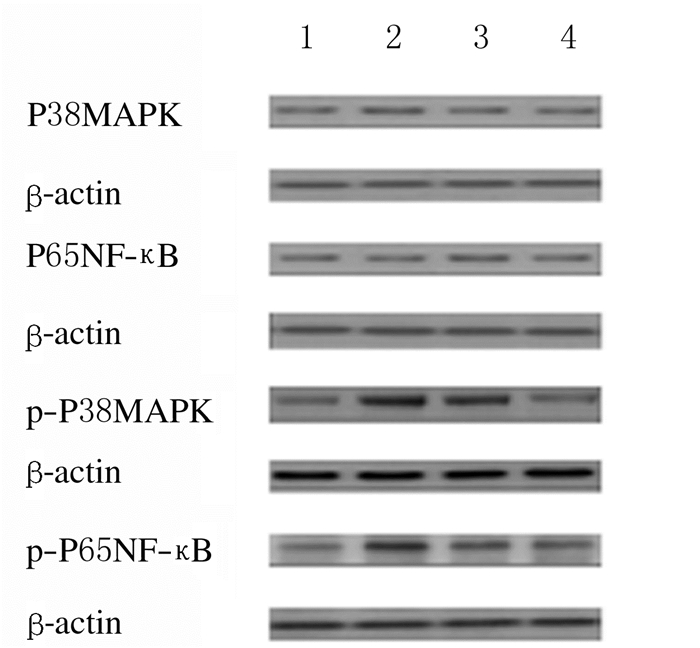
|
| Lane 1: Control group; Lane 2: Heat stroke 1 h group; Lane 3: Heat stroke 6 h group; Lane 4: Heat stroke 24 h group. 图 2 各组小鼠出仓后大脑皮层组织中P38MAPK、P65NF-κB、p-P38MAPK和p-P65NF-κB蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of P38MAPK, P65NF-κB, p-P38MAPK, and p-P65NF-κB proteins in cerebral cortex tissue of mice after leaving warehouse |
|
|
热射病的主要变化为体温快速升高超过40℃,皮肤干燥、灼热,伴有中枢神经系统损伤,严重患者可出现弥漫性血管内凝血和全身炎症反应综合征等;如果未得到及时控制,可迅速发展为急性肝损伤、肾损伤和呼吸窘迫综合征等,进一步发展为多器官功能障碍综合征,死亡率较高[4]。热射病中枢神经损伤的机制比较复杂,可能与下列因素有关:发生热射病时的高温直接损伤中枢神经系统,引起脑细胞发生一系列病理反应,导致脑水肿和代谢紊乱等,进一步发展引起脑细胞变性坏死;热射病时脑缺血、缺氧是脑损伤的主要病理生理基础,高温引起的热应激可导致脑血流量减少,在高温、缺氧的刺激下中枢神经细胞快速死亡[5-6]。除高热以及缺血、缺氧等因素外,炎症反应在热射病脑损伤中也发挥重要作用[7]。刘军等[8]研究显示:热射病小鼠中枢和外周炎症细胞因子水平升高;赵琳等[9]研究显示:热射病大鼠血清中TNF-α、IL-1β和IL-6水平升高,认为热射病的全身炎症反应激活。本研究结果显示:热射病小鼠出舱后1 h大脑皮层中大量脑细胞核固缩、染色深,血管被压缩变形、管壁外大量水肿,大脑皮层组织中IL-1β、IL-6和TNF-α水平明显升高,随后核固缩、染色深脑细胞及脑水肿逐渐恢复,IL-1β、IL-6和TNF-α水平逐渐降低,出舱24 h恢复正常。本研究结果与上述研究结果一致,表明热射病小鼠存在中枢神经系统炎症反应。
MAPK是含有丝氨酸/苏氨酸的蛋白激酶,其信号转导通路中细胞外信号调节蛋白激酶、c-JNK氨基末端激酶和P38MAPK为关键分子,与细胞中转录因子和磷酸化酶共同作用参与细胞信号转导[10]。P38MAPK相对分子质量为38 000,是MAPK的一个亚型,与炎症反应关系密切,炎症因子和应激反应等多种刺激均可激活P38MAPK,进而活化P38MAPK信号通路[11]。P38MAPK是MAPK家族中最重要的控制炎症反应成员[12]。门凌等[13]认为其信号通路通过调节炎性因子的表达参与糖尿病肾病的进程,表明p38MAPK在炎症反应中发挥重要作用。NF-κB为一种核蛋白因子,是P38MAPK下游信号分子,包括p65、c-Rel、RelB、p50和p525种亚型,各亚型之间相互组合形成二聚体[14-16];p65/p50是最常见的二聚体,与IL-1β和TNF-α介导炎症基因的激活关系密切,NF-κB激活的增加可放大炎症应答反应[17-19];NF-κB主要分布在神经元、小胶质细胞、星形胶质细胞和少突胶质细胞中,P38MAPK是NF-κB的激活途径之一,激活的P38MAPK活化NF-κB进入细胞核,引起正反馈式的炎症级联反应,从而加重组织损伤[18-21]。NF-κB通常以p65/p50二聚体的形式结合其抑制性蛋白,使其处于非活化状态,在刺激因子的作用下引起NF-κB活化,诱导多种基因表达,从而产生多种炎症因子参与炎症反应[22-23]。热射病小鼠中枢神经系统炎症反应是否与P38MAPK/P65NF-κB信号通路有关尚不清楚。本研究结果显示:热射病小鼠出舱1 h后大脑皮层组织中P38MAPK和P65NF-κB蛋白表达水平变化不明显,p-P38MAPK和p-P65NF-κB蛋白表达水平升高,出舱6和24 h逐渐降低,出舱24 h降至正常水平,表明热射病小鼠出舱后在中枢发生炎症反应的同时,P38MAPK/P65NF-κB信号通路被激活。热射病的热刺激以及高温引起的大脑缺血缺氧激活p38MAPK通路,产生IL-1β、IL-6和TNF-α等炎性细胞因子引起神经系统损伤;P38MAPK的激活进一步活化神经元中NF-κB,活化的NF-κB进入细胞核,与靶基因位点结合,诱导促炎因子靶基因转录,促进炎症细胞浸润,引起正反馈炎症级联反应,进一步加重神经系统损伤。
| [1] | LI Z, LIU P, ZHANG H, et al. Role of GABABreceptors andp38MAPK/NF-κB pathway in paclitaxel-induced apoptosis of hippocampalneurons[J]. Pharm Biol, 2017, 55(1): 2188–2195. DOI:10.1080/13880209.2017.1392987 |
| [2] | YANG W, LI J, SHANG Y, et al. HMGB1-TLR4 axis plays a regulatory role in the pathogenesis of mesial temporal lobe epilepsy in immature rat model and children via thep38MAPKsignaling pathway[J]. Neurochem Res, 2017, 42(4): 1179–1190. DOI:10.1007/s11064-016-2153-0 |
| [3] | BRUCHIM Y, GINSBURG I, SEGEV G, et al. Serum histones as biomarkers of the severity ofheatstrokein dogs[J]. Cell Stress Chaperones, 2017, 22(6): 903–910. DOI:10.1007/s12192-017-0817-6 |
| [4] | NAVARRO C S, CASA D J, BELVAL L N, et al. Exertionalheat stroke[J]. Curr Sports Med Rep, 2017, 16(5): 304–305. DOI:10.1249/JSR.0000000000000403 |
| [5] | BRUCHIM Y, HOROWITZ M, AROCH I. Pathophysiology of heat stroke in dogs-revisited[J]. Temperature (Austin), 2017, 4(4): 356–370. DOI:10.1080/23328940.2017.1367457 |
| [6] | RAUKAR N, LEMIEUX R S, CASA D J, et al. Dead heat:Treating exertional heat stroke is a race against time and temperature[J]. JEMS, 2017, 42(5): 54–59. |
| [7] | CHEN F, LI H, ZHU G, et al. Sodium tanshinone ⅡA sulfonate improves inflammation, aortic endothelial cell apoptosis, disseminated intravascular coagulation and multiple organ damage in a rat heat stroke model[J]. Mol Med Rep, 2017, 16(1): 87–94. DOI:10.3892/mmr.2017.6573 |
| [8] | 刘军, 王宫, 何根林, 等. 热射病小鼠早期中枢神经炎症和外周炎症的变化[J]. 第三军医大学学报, 2017, 39(4): 311–316. |
| [9] | 赵琳, 李军. 热射病模型大鼠凝血功能、炎症反应程度及脑组织内细胞凋亡的评估[J]. 海南医学院学报, 2017, 23(3): 303–305, 309. |
| [10] | HAN J, ZHAO Q, BASMADJIAN C, et al. Epithelial cell apoptosis and mitochondrial dysfunction[J]. Inflamm Bowel Dis, 2016, 22(1): 55–67. |
| [11] | SUN Y, GE X, LI M, et al. Dyrk2 involved in regulating LPS-induced neuronal apoptosis[J]. Int J Biol Macromol, 2017, 104(Pt A): 979–986. |
| [12] | BADSHAH H, ALI T, SHAFIQ-UR REHMAN, et al. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain[J]. J Neuroimmun Pharmacol, 2016, 11(1): 48–60. DOI:10.1007/s11481-015-9623-z |
| [13] | 门凌, 何日明, 杨曙东, 等. p38 MAPK介导炎症在糖尿病肾病中作用研究进展[J]. 陕西医学杂志, 2017, 46(7): 983–985. DOI:10.3969/j.issn.1000-7377.2017.07.058 |
| [14] | ZHANG J, ZHANG Z, XIANG J, et al. Neuroprotective Effects of echinacoside on regulating the stress-active p38MAPK and NF-κB p52 signals in the mice model of Parkinson's disease[J]. Neurochem Res, 2017, 42(4): 975–985. DOI:10.1007/s11064-016-2130-7 |
| [15] | CUI Y M, HAN X H, LIN Y Y, et al. TNF-α was involved in calcium hydroxide-promoted osteogenic differentiation of human DPSCs through NF-κB/p38MAPK/Wnt pathway[J]. Pharmazie, 2017, 72(6): 329–333. |
| [16] | LIU Y F, BAI Y Q, QI M. Daidzein attenuates abdominal aortic aneurysm through NF-κB, p38MAPK and TGF-β1 pathways[J]. Mol Med Rep, 2016, 14(1): 955–962. DOI:10.3892/mmr.2016.5304 |
| [17] | YOUSIF N G, HADI N R, AL-AMRAN F, et al. Cardioprotective effects of irbesartan in polymicrobial sepsis:The role of the p38MAPK/NF-κB signaling pathway[J]. Herz, 2018, 43(2): 140–145. DOI:10.1007/s00059-017-4537-6 |
| [18] | CAI Y, LI W, TU H, et al. Curcumolide reduces diabetic retinal vascular leukostasis and leakage partly via inhibition of the p38MAPK/NF-κB signaling[J]. Bioorg Med Chem Lett, 2017, 27(8): 1835–1839. DOI:10.1016/j.bmcl.2017.02.045 |
| [19] | YU X, CUI L, HOU F, et al. Angiotensin-converting enzyme 2-angiotensin (1-7)-Mas axis prevents pancreatic acinar cell inflammatory response via inhibition of the p38 mitogen-activated protein kinase/nuclear factor-κB pathway[J]. Int J Mol Med, 2018, 41(1): 409–420. |
| [20] | ZHANG W L, CAO Y A, XIA J, et al. Neuroprotective effect of tanshinone ⅡA weakens spastic cerebral palsy through inflammation, p38MAPK and VEGF in neonatal rats[J]. Mol Med Rep, 2018, 17(1): 2012–2018. |
| [21] | ZAKI O S, SAFAR M M, AIN-SHOKA A A, et al. Bone marrow mesenchymal stem cells combat lipopolysaccharide-induced sepsis in rats via amendment of P38-MAPK signaling cascade[J]. Inflammation, 2018, 41(2): 541–554. DOI:10.1007/s10753-017-0710-6 |
| [22] | LIU J, LIU M, WANG S, et al. Alantolactone induces apoptosis and suppresses migration in MCF 7 human breast cancer cells via the p38 MAPK, NF-κB and Nrf2 signaling pathways[J]. Int J Mol Med, 2018, 42(4): 1847–1856. |
| [23] | PANG W, QI X, CAO C, et al. Inhibitory effects of TGP on KGF induced hyperproliferation of HaCaT cells via suppression of the p38 MAPK/NF κB p65 pathway[J]. Mol Med Rep, 2018, 18(2): 2207–2215. |
 2019, Vol. 45
2019, Vol. 45


