扩展功能
文章信息
- 刘建明, 刘宸辰, 刘新民, 曾明, 蒋群
- LIU Jianming, LIU Chenchen, LIU Xinmin, ZENG Ming, JIANG Qun
- 白屈菜赤碱对大鼠肺组织的长期毒性作用及其对肺组织中NF-κB表达的影响
- Long term toxicity of chelerythrine on lung tissue of rats and its effect on expression of NF-κB in lung tissue
- 吉林大学学报(医学版), 2019, 45(03): 518-523
- Journal of Jilin University (Medicine Edition), 2019, 45(03): 518-523
- 10.13481/j.1671-587x.20190309
-
文章历史
- 收稿日期: 2018-05-27
2. 中南大学湘雅护理学院, 湖南 长沙 410013;
3. 中南大学湘雅公共卫生学院毒理教研室, 湖南 长沙 410078;
4. 中南大学湘雅二医院眼科, 湖南 长沙 410011
2. Xiangya School of Nursing, Central South University, Changsha 410013, China;
3. Department of Health Toxicology, Xiangya School of Public Health, Central South University, Changsha 410078, China;
4. Department of Ophthalmology, Second Xiangya Hospital, Central South University, Changsha 410011, China
白屈菜赤碱是一种苯并菲啶类生物碱,对包括鼻咽癌、宫颈癌、胃癌和肺癌在内的多种肿瘤细胞具有杀伤作用[1-2];其可通过凋亡因子抑制细胞周期,诱导肿瘤细胞凋亡,产生氧自由基和抑制蛋白激酶C的活性,发挥抗肿瘤作用[3-5]。体内研究[6-7]表明:白屈菜赤碱可以改善大鼠肝纤维化。急性毒性实验中,白屈菜赤碱可引起大鼠急性呼吸窘迫,有发生死亡的危险,显示很强的毒性反应。长期毒性实验未见大鼠骨髓抑制,但可引起雄性大鼠睾丸病理损伤、肺栓塞和血性腹水;当白屈菜赤碱剂量超过5.6 mg·kg-1时可引起全身反应[8]。
炎症级联反应在肺组织损伤和病理过程中起关键作用。肺泡上皮细胞、炎性细胞和成纤维细胞能产生多种细胞因子参与炎症反应,可加重炎症损伤。核转录因子κB(nuclear factor kappa beta,NF-κB)、p38丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路是主要的转导途径。NF-κB具有多种调节功能[9-11],能介导各种炎症介质、细胞因子和趋化因子的基因转录水平,如白细胞介素8(interleukin-8,IL-8)、白细胞介素6(interleukin-6, IL-6)、细胞间黏附分子1(intercellular adhesion molecule-1, ICAM-1)和肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)等,在细胞的炎症和凋亡中起重要作用。已有研究[12]表明NF-κB参与了肺组织损伤。中性粒细胞可黏附于损伤的血管内皮细胞,并向肺泡腔和肺间质转移,从而产生大量炎症介质,如白三烯和炎性细胞因子等,参与中性粒细胞诱导的肺损伤。ICAM-1参与炎症反应及相关病理过程,多种炎症因子可提高其表达水平[13-14]。目前有关白屈菜赤碱的药效学和毒理学实验的研究报道较少。研究[15]显示:白屈菜赤碱对细胞周期的生长有明显作用,能抑制肿瘤细胞的生长,但也会引起全身性异常反应,甚至死亡,其相关机制尚不清楚。为了进一步探讨白屈菜赤碱的毒性作用及其对靶器官的影响,本研究观察白屈菜赤碱对大鼠肺组织损伤和肺组织中NF-κB及ICAM-1mRNA表达水平的影响,探讨其对大鼠肺组织长期毒性作用及其相关机制。
1 材料与方法 1.1 主要试剂和仪器白屈菜赤碱(浓缩液,0.01 g·L-1,0.25L)由欧化药业提供,聚氨酯购自美国Sigma公司,IL-6和IL-8检测试剂盒由南京建成生物科技公司提供,NF-κB和ICAM-1引物由美国Invitrogen公司提供,其蛋白抗体、二抗、反转录试剂盒和PCR扩增试剂盒购自北京中杉金桥生物科技公司,Western blotting实验试剂购于上海Beyotime公司,其他常见试剂购于上海生工公司。酶标仪和PCR仪为美国ABI产品,光学显微镜(CX41)购于日本Olympus公司。
1.2 实验动物分组及处理健康Wistar大鼠120只,雌雄各半,体质量120~140 g,由中南大学实验动物中心提供,动物合格证号:SYXK(湘)2015-0017,SPF级,饲养于屏障环境。根据急性毒性实验结果确定半数抑制浓度(IC50)为24.5 mg·kg-1,并结合相关文献[8](有效剂量2.0 mg·kg-1)确定白屈菜赤碱的给药剂量。将大鼠随机分为对照组和低(3.7 mg·kg-1)、中(5.6 mg·kg-1)及高剂量(8.4 mg·kg-1)白屈菜赤碱组,每组30只。根据抗肿瘤药物细胞毒性研究指南[12-13]给药,连续腹腔注射给药6 d,间隔8 d,再注射给药6 d,间隔8 d,反复3次,停药后连续监测4周。观察各组大鼠一般情况,记录大鼠累积死亡率。每次药物注射量为1 mL·100 g-1。对照组大鼠给予等体积生理盐水。
1.3 酶联免疫吸附(ELISA)法测定各组大鼠血清IL-6、IL-8和TNF-α水平按照试剂盒说明书检测各组大鼠血清中IL-6、IL-8和TNF-α水平。
1.4 光学显微镜观察各组大鼠肺组织形态表现处死大鼠,立刻取出肺组织,观察大体形态,采用甲醛浸泡,石蜡包埋,制备成厚度5 μm切片,经苏木精-伊红(HE)染色后,光学显微镜下观察各组大鼠肺组织形态表现。
1.5 实时荧光定量PCR法检测各组大鼠肺组织中NF-κB和ICAM-1 mRNA表达水平将肺组织标本制成匀浆。采用TransZol法测量RNA总量。将反转录的cDNA作为模板进行PCR扩增。PCR反应步骤:95℃预变性10 min,共40个循环,包括95℃变性10 s,退火55℃、30 s,72℃延伸45 s。以β肌动蛋白(β-actin)为内参照物,采用Rotor-Gene Q系列软件进行半定量分析。引物序列见表 1。
| Primer | Size(bp) | Forward | Reverse |
| NF-κB | 100 | 5′-GAGAGCCCTTGCATCCTTTA-3′ | 5′-CTTCCCTTTGGTCTTTCTGT-3′ |
| ICAM-1 | 260 | 5′-CGACTGGACGAGAGGGATTG-3′ | 5′-TTATGACTGCGGCTGCTACC-3′ |
| β-actin | 220 | 5′-TGCTGTCCCTGTATGCCTCT-3′ | 5′-TTTGATGTCACGCACGATTT-3′ |
采用RIPA裂解液提取100 mg肺组织标本中的蛋白,采用Bradford法测定蛋白浓度,SDS-PAGE凝胶电泳分离蛋白质,转移到聚偏二氟乙烯膜。加入抗NF-κB和ICAM-1单克隆抗体(1:100稀释)孵育过夜。缓冲液漂洗,加入二抗(1:200稀释)孵育1 h。采用化学发光剂显影,采用成像分析软件对Western blotting条带进行分析,以条带灰度值表示蛋白表达水平。实验重复3次。
1.7 统计学分析采用SPSS 19.0统计软件进行统计学分析。各组大鼠血清IL-6、IL-8和TNF-α水平及肺组织中NF-κB和ICAM-1mRNA和蛋白表达水平以x±s表示, 组间比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠一般情况给予白屈菜赤碱后,高剂量白屈菜赤碱组大鼠立即出现痉挛和腹膜刺激症现象,长达40 min。第一轮暴露结束后,大鼠逐渐出现腹水和肺充血。各剂量白屈菜赤碱组大鼠体质量和摄食量均明显低于对照组(P < 0.05),高剂量白屈菜赤碱组大鼠体质量和摄食量低于低和中剂量白屈菜赤碱组(P < 0.05)。高剂量白屈菜赤碱组大鼠累积死亡率最高,其次是中和低剂量组。见图 1。
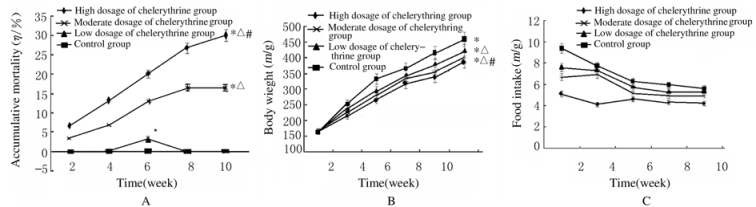
|
| *P < 0.05 compared with control group; △P < 0.05 compared with low dosage of chelerythrine group; #P < 0.05 compared with moderate dosage of chelerythrine group. 图 1 各组大鼠累积死亡率(A)、体质量(B)和摄食量(C) Fig. 1 Accumulative mortalities(A), body weights(B), and food intakes(C) of rats in various groups |
|
|
与对照组比较,各剂量白屈菜赤碱组大鼠血清IL-6、IL-8和TNF-α水平升高(P<0.05)。随着白屈菜赤碱剂量的增加,其水平升高呈剂量依赖性。见图 2。
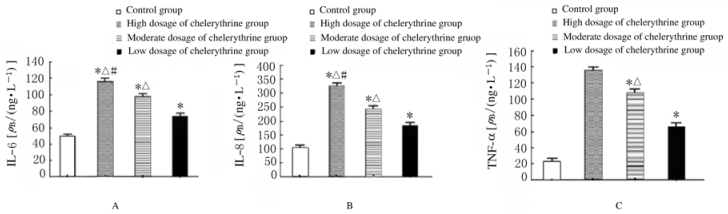
|
| *P < 0.05 compared with control group; △P < 0.05 compared with low dosage of chelerythrine group; #P < 0.05 compared with moderate dosage of chelerythrine group. 图 2 ELISA法检测各组大鼠血清IL-6(A)、IL-8(B)和TNF-α(C)水平 Fig. 2 Levels of IL-6(A), IL-8 (B), and TNF-α(C) in serum of rats in various groups detected by ELISA method |
|
|
HE染色结果显示:对照组大鼠肺组织无明显异常,而随着白屈菜赤碱剂量的增加,各剂量白屈菜赤碱组大鼠肺组织损伤加重,停药后大鼠损伤的肺组织未完全恢复。低、中和高剂量白屈菜赤碱组大鼠在给药期间均出现炎性浸润。高剂量白屈菜赤碱组大鼠肺泡壁增厚,中和低剂量白屈菜赤碱组大鼠显示受损程度减轻。见图 3(插页三)。
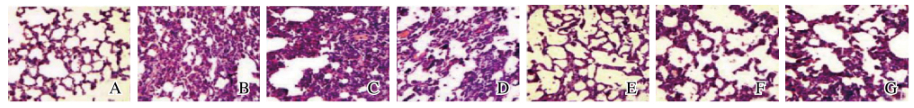
|
| A:Control group;B:High dosage of chelerythrine group;C:Moderate dosage of chelerythrine group;D:Low dosage of chelerythrine group;B,D,F:Drug delivery period;C,E,G;Recovery period。 图 3 各组大鼠肺组织形态表现(HE,×100) Fig. 3 Morphology of lung tissue of rats in various groups(HE,×100) |
|
|
与对照组比较,各剂量白屈菜赤碱组大鼠肺组织中NF-κB和ICAM-1 mRNA和蛋白表达水平均升高(P<0.05),且呈剂量依赖性。见图 4和5。
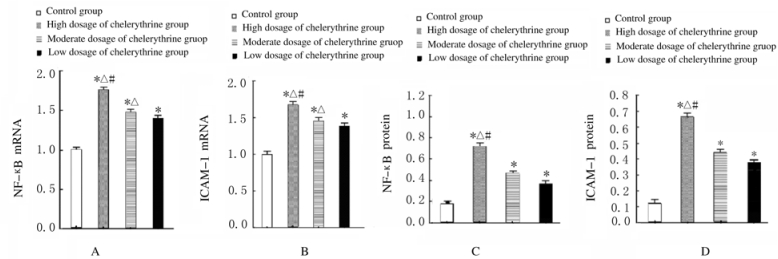
|
| A:NF-κB mRNA; B:ICAM-1 mRNA; C:NF-κB protein; D:ICAM-1 protein.*P < 0.05 compared with control group; △P < 0.05 compared with low dosage of chelerythrine group; #P < 0.05 compared with moderate dosage of chelerythrine group. 图 4 实时荧光定量PCR法检测各组大鼠肺组织中NF-κB和ICAM-1 mRNA表达水平 Fig. 4 Expression levels of NF-κB and ICAM-1 mRNA in lung tissue of rats in various groups detected by real-time fluorescence quantitative PCR method |
|
|

|
| Lane 1:Control group; Lane 2:High dosage of chelerythrine group; Lane 3:Moderate dosage of chelerythrine group; Lane 4:Low dosage of chelerythrine group. 图 5 Western blotting法检测各组大鼠肺组织中NF-κB和ICAM-1蛋白表达电泳图 Fig. 5 Electrophoretogram of expressions of NF-κB and ICAM-1 proteins in lung tissue of rats in various groups detected by Western blotting method |
|
|
本研究观察白屈菜赤碱对大鼠及其靶器官长期毒性作用的结果表明:连续注射白屈菜赤碱对大鼠肺组织造成一定的损害,表现为肺充血和血性腹水。高剂量白屈菜赤碱组大鼠肺组织损伤程度加重,停止染毒后不能完全恢复。表明随着白屈菜赤碱剂量的增加,其对肺组织存在长期毒性作用,随着剂量(≥5.6 mg·kg-1)增加,其可引起与药物毒性有关的肺损伤,尤其在高剂量白屈菜赤碱组。炎症损伤在急性和慢性肺组织损伤中起关键作用。IL-6主要来源于单核巨噬细胞、内皮细胞、血管平滑肌细胞和T淋巴细胞,并能促进肺组织中ICAM-1基因的表达。上调的ICAM-1 mRNA能与中性粒细胞表面蛋白LFA-1结合,启动中性粒细胞与内皮细胞之间信号转导,促进黏附,诱导中性粒细胞浸润[16]。
IL-8是T淋巴细胞和中性粒细胞的炎症细胞趋化因子,主要来源于上皮细胞、巨噬细胞和中性粒细胞。IL-6能刺激IL-8的产生,后者可与细胞表面影响细胞信号转导的G蛋白偶联受体(GPCR)结合,诱导白细胞黏附和活化,促进白细胞与胞外基质(ECM)蛋白结合以释放蛋白质水解酶和过氧化物,从而引起肺组织损伤[17-18]。本研究结果显示:随着白屈菜赤碱剂量的增加,大鼠血清IL-6和IL-8水平升高,表明白屈菜赤碱的长期毒性作用可引起大鼠血清IL-6和IL-8水平升高,促进炎性损伤。
许多细胞信号通路调节炎症介质的产生和释放,包括IL-6和IL-8。NF-κB是急性肺损伤的一种重要途径[19]。体内和体外实验均显示NF-κB参与肺组织损伤的病理过程[20]。胞浆内NF-κB无活性,与抑制蛋白IκB以结合形式存在。NF-κB激活以后,促进IκB表达,后者又对NF-κB进行反馈调节,进一步促进NF-κB表达[21]。IL-1和脂多糖(LPS)信号导致IκB降解,引起NF-κB进一步释放和活化,进入细胞核与κB特异结合,促进炎症介质和细胞因子的转录,产生炎性因子和炎症介质,从而引起炎症反应。急性炎性损伤早期的关键步骤可能与磷酸化后IκB的降解及复合物中NF-κB移位有关[20-22]。
NF-κB结合序列在ICAM-1和IL-8基因上游启动子和增强子中大量表达,其上调可激活相关基因的表达水平[23-25]。ICAM-1是LFA-1的配体,能促进内皮细胞和白细胞之间的黏附作用。但目前有关肺组织损伤与NF-κB和ICAM-1在肺组织中的表达水平关系的研究未见报道。因此,本研究重点探讨了白屈菜赤碱长期毒性对NF-κB和ICAM-1在大鼠肺组织中表达的影响,结果显示:剂量递增方式连续给药,大鼠肺组织中ICAM-1和NF-κB mRNA表达水平升高,表明白屈菜赤碱能增强NF-κB mRNA的表达,促进白细胞和内皮细胞黏附作用或通过增强炎性因子的表达增强炎症反应。逐步上调NF-κB和ICAM-1蛋白表达水平会进一步加重肺组织损伤。长期注射白屈菜赤碱对肺组织有一定的毒性,其机制可能与NF-κB信号通路有关,可通过该通路促进炎症因子的表达而引起炎症损伤。但本研究只涉及白屈菜赤碱对大鼠肺组织形态学长期毒性作用和相关的NF-κB信号通路,未探讨该信号通路详细的机制及其对其他信号通路可能的影响,有待于未来进一步研究。
| [1] | DELJANIN M, NIKOLIC M, BASKIC D, et al. Chelidonium majus crude extract inhibits migration and induces cell cycle arrest and apoptosis in tumor cell lines[J]. J Ethnopharmacol, 2016, 190(16): 362–371. |
| [2] | CAPISTRANO I R, WOUTERS A, LARDON F, et al. In vitro and in vivo investigations on the antitumour activity of Chelidonium majus[J]. Phytomedicine, 2015, 22(14): 1279–1287. DOI:10.1016/j.phymed.2015.10.013 |
| [3] | PARK S W, KIM S R, KIM Y, et al. Extract induces apoptosis through caspase activity via MAPK-independent NF-kappaB signaling in human epidermoid carcinoma A431 cells[J]. Oncol Rep, 2015, 33(1): 419–424. DOI:10.3892/or.2014.3566 |
| [4] | 张步鑫, 赵献敏, 成琼, 等. 白屈菜红碱对黑色素瘤B16细胞增殖抑制和凋亡诱导作用的实验研究[J]. 时珍国医国药, 2018, 29(4): 793–795. |
| [5] | BAEK S C, RYU H W, KANG M G, et al. Selective inhibition of monoamine oxidase A by chelerythrine, an isoquinoline alkaloid[J]. Bioorgan Med Chem Lett, 2018, 28(14): 2403–2407. DOI:10.1016/j.bmcl.2018.06.023 |
| [6] | 李晓明, 欧阳婷庭, 董妙先, 等. 白屈菜红碱对肝纤维化小鼠TGF-β/Smads信号通路的影响[J]. 中国病理生理杂志, 2018, 34(7): 1323–1328. DOI:10.3969/j.issn.1000-4718.2018.07.028 |
| [7] | EI-READI M Z, EID S, ASHOUR M L, et al. Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids[J]. Phytomedicine, 2013, 20(3): 282–294. |
| [8] | BANERJEE A, PATHAK S, BISWAS S J, et al. Chelidonium majus 30C and 200C in induced hepato-toxicity in rats[J]. Homeopathy, 2010, 99(3): 167–176. DOI:10.1016/j.homp.2010.05.008 |
| [9] | USMAN H A, HERNOWO B S, TOBING M D, et al. The major role of nf-kappa b in the depth of invasion on acral melanoma by decreasing cd8(+) t cells[J]. J Pathol Translat Med, 2018, 52(3): 164–170. DOI:10.4132/jptm.2018.04.04 |
| [10] | CHO S O, KIM M H, KIM H. beta-Carotene inhibits activation of NF-kappaB, activator protein-1, and STAT3 and regulates abnormal expression of some adipokines in 3T3-L1 adipocytes[J]. J Cancer Prevent, 2018, 23(1): 37–43. DOI:10.15430/JCP.2018.23.1.37 |
| [11] | LEE Y K, YI E Y, PARK S Y, et al. Mitochondrial dysfunction suppresses p53 expression via calcium-mediated nuclear factor-kB signaling in HCT116 human colorectal carcinoma cells[J]. Bmb Rep, 2018, 51(6): 296–301. DOI:10.5483/BMBRep.2018.51.6.232 |
| [12] | KOLAC U K, USTUNER M C, TEKIN N. The anti-inflammatory and antioxidant effects of salvia officinalis on lipopolysaccharide-induced inflammation in rats[J]. J Med Food, 2017, 20(12): 1193–1120. DOI:10.1089/jmf.2017.0035 |
| [13] | SHEN H, SHENG L, XIONG Y, et al. Thymic NF-kappaB-inducing Kinase (NIK) regulates CD4+ T cell-elicited liver injury and fibrosis in mice[J]. J Hepatol, 2017, 67(1): 100–109. DOI:10.1016/j.jhep.2017.02.025 |
| [14] | ZHANG R, AI X, DUAN Y, et al. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-kappaB and MAPK signaling pathways[J]. Biomed Pharmacother, 2017, 89: 660–672. DOI:10.1016/j.biopha.2017.02.081 |
| [15] | 罗飞亚, 马新群, 林飞, 等. 白屈菜红碱对大鼠的长期毒性试验研究[J]. 癌变·畸变·突变, 2014, 26(6): 459–462. DOI:10.3969/j.issn.1004-616x.2014.06.013 |
| [16] | KORIEM K M, ARBID M S, ASAAD G F. Chelidonium majus leaves methanol extract and its chelidonine alkaloid ingredient reduce cadmium-induced nephrotoxicity in rats[J]. J Nat Med, 2013, 67(1): 159–167. DOI:10.1007/s11418-012-0667-6 |
| [17] | PAUL A, DAS J, DAS S, et al. Poly (lactide-co-glycolide) nano-encapsulation of chelidonine, an active bioingredient of greater celandine (Chelidonium majus), enhances its ameliorative potential against cadmium induced oxidative stress and hepatic injury in mice[J]. Environ Toxicol Pharmacol, 2013, 36(3): 937–947. DOI:10.1016/j.etap.2013.08.008 |
| [18] | SAMANTA J, SINGH S, ARORA S, et al. Cytokine profile in prediction of acute lung injury in patients with acute pancreatitis[J]. Pancreatology, 2018, 18(8): 878–884. DOI:10.1016/j.pan.2018.10.006 |
| [19] | ZENG M C, SANG W H, CHEN S, et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models[J]. Toxicol Lett, 2017, 271: 26–37. DOI:10.1016/j.toxlet.2017.02.023 |
| [20] | WANG T, HOU W R, FU Z. Preventative effect of OMZ-SPT on lipopolysaccharide-induced acute lung injury and inflammation via nuclear factor-kappa B signaling in mice[J]. Biochem Biophys Res Commun, 2017, 485(2): 284–289. DOI:10.1016/j.bbrc.2017.02.090 |
| [21] | HUANG C, PAN L, LIN F, et al. Monoclonal antibody against Toll-like receptor 4 attenuates ventilator-induced lung injury in rats by inhibiting MyD88- and NF-κB-dependent signaling[J]. Int J Mol Med, 2017, 39(3): 693–700. DOI:10.3892/ijmm.2017.2873 |
| [22] | LU X G, PU Y X, KONG W G, et al. Antidesmone, a unique tetrahydroquinoline alkaloid, prevents acute lung injury via regulating MAPK and NF-κB activities[J]. Int Immunopharmacol, 2017, 45(4): 34–42. |
| [23] | SHIN W G, PARK B J, LEE S J, et al. Infection of human intestinal epithelial cells by invasive bacteria activates NF-kappa B and increases ICAM-1 expression through NOD1[J]. Korean J Int Med, 2018, 33(1): 81–90. DOI:10.3904/kjim.2015.409 |
| [24] | KIM S H, PAEK Y W, KANG I C. Inhibition of lipopolysaccharide-stimulated inflammatory cytokine production by LY303511 in human macrophagic THP-1 cells[J]. Internat J Oral Biol, 2017, 42(3): 117–121. DOI:10.11620/IJOB. |
| [25] | 张明, 唐晶晶, 谢颖颖, 等. 姜黄素对慢性阻塞性肺疾病大鼠肺泡上皮细胞内质网应激的影响[J]. 西安交通大学学报:医学版, 2018, 39(3): 361–365. |
 2019, Vol. 45
2019, Vol. 45


