扩展功能
文章信息
- 王天玥, 周倩兰, 尚云晓
- WANG Tianyue, ZHOU Qianlan, SHANG Yunxiao
- 表没食子儿茶素没食子酸酯对肥胖型哮喘小鼠气道炎症和Treg/Th17免疫平衡的影响
- Effect of epigallocatechin-3-gallate on airway inflammation and Treg/Th17 immune balance of mice with obese asthma
- 吉林大学学报(医学版), 2019, 45(03): 491-497
- Journal of Jilin University (Medicine Edition), 2019, 45(03): 491-497
- 10.13481/j.1671-587x.20190305
-
文章历史
- 收稿日期: 2018-09-12
肥胖的发生不但在全球范围内呈现上升趋势,在儿童和青少年人群中也相当普遍。肥胖和哮喘患病率增加存在并行现象,均属于多因素复杂疾病类型,二者在发生发展中相互影响,因此在肥胖和哮喘交互作用方面的研究也逐渐增多[1]。哮喘是气道慢性炎症疾病,而肥胖型哮喘是哮喘表型中一种难治的亚型,在2014年版的全球哮喘防治创议(Global Initiative for Asthma, GINA)方案中被首次提出[2]。临床上肥胖型哮喘患者常常表现为哮喘症状重,且对于糖皮质激素及长效β2受体激动剂等常规治疗不敏感,症状难以控制[3]。
经典免疫学说认为哮喘的发病机制主要与辅助性T淋巴细胞1/辅助性T淋巴细胞2(helper T lymphocyte 1/ helper T lymphocyte 2, Th1/Th2)功能失衡有关,但这种理论并不适用于肥胖型哮喘。近年研究[4-5]表明:在哮喘中除Th1/Th2免疫失衡外,亦存在调节性T淋巴细胞/辅助性T淋巴细胞17(regulatory T lymphocyte/helper T lymphocyte 17, Treg/Th17)免疫失衡,Treg和Th17在哮喘发生发展的病理过程中发挥重要作用。肥胖型哮喘的发病机制更为复杂,尚不清楚。研究[6]表明:在肥胖小鼠肺组织中Th17增多,Th17分泌的白细胞介素17A(interleukin-17A,IL-17A)能够诱发肥胖小鼠的气道高反应性;在儿童中进行的研究[7]表明:与正常儿童比较,肥胖患儿以及肥胖型哮喘患儿外周血中的Treg数量均降低, 以上研究提示:在肥胖型哮喘这一难治性的哮喘亚型中,很可能存在Th17介导的中性粒细胞气道炎症,存在Treg/Th17免疫失衡。IL-17为Th17分泌的一种细胞因子,也是诱发中性粒细胞募集及活化的重要启动因子,因此IL-17水平可以反映中性粒细胞炎症的浸润程度[8]。中性粒细胞浸润为主导的哮喘类型一直被认为与哮喘的急性发作、控制不佳和重症哮喘有密切的关系,并且其对于激素治疗效果不理想,甚至出现激素抵抗[9-10]。
表没食子儿茶素没食子酸酯(epigallocatechin gallate,EGCG)是一种绿茶提取物,是绿茶中生物活性最强的儿茶素,学者们发现其不仅有抗氧化的作用,还有抗炎、抗肿瘤等多种功效。研究[11]表明:EGCG能够抑制哮喘小鼠的气道炎症反应,同时亦能够调节CD4+T细胞亚群,通过抑制Th17、增加Treg数量,从而缓解肥胖诱发的自身免疫性关节炎[12]。本研究建立肥胖型哮喘小鼠模型,采用EGCG进行干预,探讨其对肥胖型哮喘小鼠气道炎症及Treg/Th17免疫平衡的影响,为EGCG治疗肥胖型哮喘提供依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器40只健康雌性SPF级C57BL/6J小鼠,4周龄,由中国医科大学附属盛京医院动物实验室提供,动物生产许可证号:SCXK(辽)2015-0001。高脂饲料D12492和正常饲料D12450B购于美国Research Diets公司,卵白蛋白(ovalbumin,OVA;GradeⅡ)和EGCG购自美国Sigma公司,氢氧化铝干粉购自沈阳化工三厂,甲基乙酰胆碱(Mch)由中国医科大学附属第一医院肺功能室惠赠,脂联素、瘦素、IL-10和IL-17的ELISA试剂盒购于美国R & D公司,CD24、CD25、IL-17A和Foxp3抗体购自美国BD公司。小动物无创肺功能检测仪为法国Emka公司生产,仪器型号为GYD-003;流式细胞仪为美国DB公司产品,仪器型号为C6。
1.2 动物分组和模型建立SPF级C57BL/6J小鼠随机分为正常对照组、肥胖组、肥胖型哮喘组、EGCG干预组和地塞米松干预组,每组8只。肥胖组、肥胖型哮喘组、EGCG干预组和地塞米松干预组小鼠均给予高脂饲料(60%脂肪热能)喂养12周,正常对照组小鼠则给予正常饲料(10%脂肪热能)喂养。实验开始第9、10和11周,肥胖型哮喘组、EGCG和地塞米松干预组小鼠每周注射1次OVA致敏液0.2 mL(即20μg卵蛋白与2 mg氢氧化铝溶于0.2 mL生理盐水中),共注射3次。第12周,肥胖型哮喘组、EGCG和地塞米松干预组小鼠以4%OVA行超声雾化吸入激发,每次30 min,每天1次,连续7 d,正常对照组和肥胖组小鼠给予生理盐水雾化。EGCG干预组小鼠在每次超声雾化前1 h给予20 mg·kg-1EGCG腹腔注射,地塞米松干预组小鼠则给予2 mg·kg-1地塞米松腹腔注射。最后一次OVA激发后48 h将小鼠处死。
1.3 各组小鼠气道反应性测定末次激发24 h后测定各组小鼠气道反应性。本实验采用小动物无创肺功能检测仪检测各组小鼠肺功能,采用体积描记法测定C57BL/6J小鼠的增强呼气间歇(enhanced pause,Penh)。将小鼠自由放置于密闭容器内,容器内的压力随着小鼠的呼吸而变化,通过测量容器内压力的变化,得到小鼠的呼吸曲线,该仪器可自动生成Penh数值。具体方法:每只小鼠轮流放入体描箱中,使其稳定3 min适应环境,依次测量生理盐水和Mch浓度为3.125、6.250、12.500、25.000和50.000 g·L-1激发时的Penh值,每次雾化吸入时间为1 min,记录3 min,基础值和每个激发剂量的Penh值取雾化后3 min的平均值。
1.4 HE染色观察各组小鼠支气管黏膜和周围肺组织形态表现HE染色,光镜下观察小鼠支气管黏膜下和周围肺组织炎性细胞浸润。采用HE染色切片半定量方法评定支气管周围炎症细胞浸润程度[13]。评分标准:0分,无炎症细胞;1分,少许炎症细胞;2分,较多分布不均的炎症细胞;3分,大量炎症细胞,分布较均匀,少见聚集成团;4分,大量炎症细胞聚集成团。
1.5 ELISA法检测各组小鼠血清中脂联素、瘦素和BALF中IL-10及IL-17A水平严格按照试剂盒说明书操作,测定各组小鼠血清中脂联素、瘦素和BALF中IL-10及IL-17A水平。
1.6 流式细胞检测各组小鼠脾组织中Treg和Th17百分比取小鼠脾脏,研磨后制成细胞悬液,200目筛网过滤,110 g离心5 min,去上清,留沉淀。PBS清洗2次,110 g离心5 min,去上清,留沉淀。加入4 mLPBS,吹悬后,110 g离心10 min,去上清,加入1 mL基础培养基重悬细胞,进行细胞计数。取出10 μL细胞稀释100倍,台盼蓝染色,显微镜下观察计数。选取1×106细胞(500 μL),分装到流式管内。除对照样本,其余样本中每管加入1 μg CD4抗体,4℃避光孵育30 min。Th17百分比检测:先标记CD4抗体后,110 g离心5 min,去上清,每组加入250 μL固定和破膜缓冲液,4℃避光孵育30 min进行破膜。110 g离心5 min,去上清,加入500 μL稀释为1×Permeabilization Buffer,洗涤细胞2次。110 g离心后弃上清,500 mL PBS重悬,再向胞内加入1 μg IL-17抗体标记。Treg百分比检测:加入1 μg CD4抗体后,再向细胞中加入1 μg CD25抗体孵育30 min。110 g离心5 min,去上清,每组加入250 μL Fixation and Permeabilization Solution,4℃避光孵育30 min进行破膜。110 g离心5 min,去上清,加入500 μL 1×Permeabilization Buffer,洗涤细胞2次。110 g离心后弃上清,500 mL PBS重悬,再向细胞中加入1 μg Foxp3抗体标记。加入2 mL PBS洗涤细胞,离心后弃上清。采用500 μL PBS重悬细胞,上机检测。
1.7 统计学分析采用SPSS 22.0统计软件进行统计学分析。各组小鼠体质量,气道阻力,气道炎症评分,血清中脂联素和瘦素水平,BALF中IL-10和IL-17A水平,脾组织中Treg和Th17百分比均以x±s表示,组间比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠体质量在小鼠处死前测量各组小鼠体质量。正常对照组小鼠体质量为(25.15±2.09) g,高脂饲料喂养的肥胖组小鼠体质量为(42.63±2.72) g,肥胖组小鼠体质量较正常对照组明显增加(P < 0.05),为正常对照组小鼠平均体质量的1.67倍;EGCG干预组小鼠体质量略低,为(39.61±2.88) g,但与肥胖型模型组小鼠的体质量比较差异无统计学意义(P>0.05)。
2.2 各组小鼠气道反应性随着给予倍增浓度的Mch,各组小鼠的Penh值逐渐升高。在给予Mch进行气道激发后,肥胖组、肥胖型哮喘组、EGCG干预组和地塞米松干预组小鼠的Penh值均高于正常对照组(P<0.05)。与肥胖组比较,肥胖型哮喘组和地塞米松干预组小鼠Penh值均升高(P<0.05);与肥胖型哮喘组比较,EGCG干预组小鼠Penh值降低(P<0.05),地塞米松干预组小鼠Penh值差异无统计学意义(P>0.05);与EGCG干预组比较,地塞米松干预组小鼠Penh值升高(P<0.05)。见表 1。
| (n=8, x±s) | |||||
| Group | Penh value | ||||
| (g·L-1)3.125 | 6.250 | 12.500 | 25.000 | 50.000 | |
| Normal control | 0.52±0.12 | 1.09±0.29 | 1.58±0.35 | 2.07±0.30 | 2.37±0.40 |
| Obese | 1.09±0.18* | 1.76±0.07* | 2.39±0.67* | 2.89±0.25* | 3.55±0.34* |
| Obese asthma | 2.57±0.29*△ | 3.53±0.20*△ | 4.28±0.34*△ | 6.05±0.70*△ | 6.57±0.81*△ |
| EGCG intervention | 2.07±0.12* | 2.66±0.50*# | 3.00±0.44*# | 3.23±0.42*# | 4.21±0.54*# |
| Dexamethasone intervention | 2.60±0.19*△ | 3.62±0.49*△○ | 4.35±0.78*△○ | 5.55±0.65*△○ | 6.04±0.95*△○ |
| * P < 0.05 compared with normal control group;△P < 0.05 compared with obese group;#P < 0.05 compared with obese asthma group;○P < 0.05 compared with EGCG intervention group. | |||||
正常对照组小鼠支气管形态规整,支气管管腔光滑,上皮细胞无明显增生,管壁无增厚;肺泡间隔正常、肺泡壁结构完整,肺泡腔和支气管内未见炎性渗出物,支气管和血管壁周围无明显炎性细胞浸润。肥胖型哮喘组可见气道黏膜水肿明显,肺泡间隔增厚,支气管壁增厚、管腔狭窄,支气管和血管壁周围可见大量炎性细胞浸润。EGCG和地塞米松干预组小鼠肺组织病理改变均有所减轻,支气管、血管周围炎症细胞浸润程度明显降低。见图 1(插页二)。与正常对照组[(0.38±0.23)分]比较,肥胖组[(1.39±0.37)分]和肥胖型哮喘组[(3.25±0.46)分]小鼠肺组织炎症评分升高(P < 0.05);与肥胖型哮喘组比较,EGCG干预组[(2.38±0.52)分]和地塞米松干预组[(2.50±0.76)分]小鼠肺组织炎症评分明显降低(P < 0.05),但高于正常对照组(P < 0.05)。
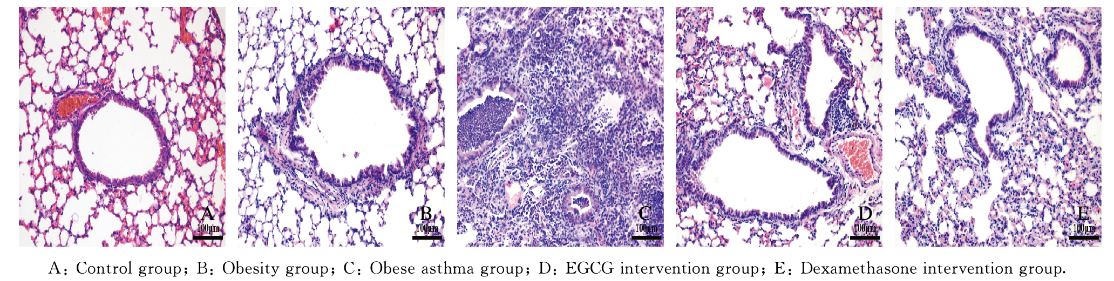
|
| 图 1 各组小鼠肺组织炎症形态表现(HE, ×200) Fig. 1 Inflammation morphology of lung tissue of mice in various groups(HE, ×200) |
|
|
与正常对照组比较,肥胖组、肥胖型哮喘组、EGCG干预组和地塞米松干预组小鼠血清中脂联素水平降低(P < 0.05)。地塞米松干预组小鼠血清中脂联素水平较肥胖型哮喘组降低(P < 0.05);EGCG干预组小鼠血清中脂联素水平与肥胖型哮喘组比较差异无统计学意义(P>0.05)。与正常对照组比较,肥胖组、肥胖型哮喘组、EGCG干预组和地塞米松干预组小鼠血清中瘦素水平均升高(P < 0.05)。与肥胖型哮喘模型组比较,EGCG干预组小鼠血清中瘦素水平降低(P < 0.05),地塞米松干预组小鼠血清中瘦素水平升高(P < 0.05)。见表 2。
| [n=8, x±s, ρB/(pg·L-1)] | ||
| Group | Adiponectin | Leptin |
| Normal control | 36.59±2.28 | 3.86±0.45 |
| Obese | 26.67±2.03* | 23.62±2.97* |
| Obese asthma | 25.33±1.75* | 21.50±2.06* |
| EGCG intervention | 27.20±3.08* | 16.85±2.44*△ |
| Dexamethasone intervention | 20.46±2.12*△ | 29.45±2.09*△ |
| * P < 0.05 compared with normal control group;△ P < 0.05 compared with obese asthma group. | ||
与正常对照组比较,肥胖组和肥胖型哮喘组小鼠BALF中IL-10水平降低(P < 0.05);EGCG干预组和地塞米松干预组小鼠BALF中IL-10水平高于肥胖型哮喘组(P < 0.05);EGCG干预组与地塞米松干预组小鼠BALF中IL-10水平比较差异无统计学意义(P>0.05)。与正常对照组比较,肥胖组和肥胖型哮喘组小鼠BALF中IL-17A水平升高(P < 0.05);EGCG干预组和地塞米松干预组小鼠BALF中IL-17A水平低于肥胖型哮喘组(P < 0.05);EGCG干预组小鼠BALF中IL-17A水平低于地塞米松干预组(P < 0.05)。见表 3。
| [n=8, x±s, ρB/(μg·L-1)] | ||
| Group | IL-10 | IL-17A |
| Normal control | 9.50±0.73 | 16.54±1.33 |
| Obese | 4.06±0.29* | 42.61±2.75* |
| Obese asthma | 2.55±0.37* | 64.92±4.07* |
| EGCG intervention | 7.13±0.55△ | 35.80±3.64*△ |
| Dexamethasone intervention | 7.26±0.68△△ | 50.88±3.79*△# |
| * P < 0.05 compared with normal control group;△ P < 0.05 compared with obese asthma group;# P < 0.05 compared with EGCG intervention group. | ||
与正常对照组(11.50%±1.10%)比较,肥胖组小鼠脾组织中Treg百分比(6.81%±0.43%)和肥胖型哮喘组(2.3%±0.37%)小鼠脾组织中Treg百分比降低(P < 0.05);肥胖型哮喘组小鼠脾组织中Treg百分比低于肥胖组(P < 0.05)。与肥胖型哮喘组比较,EGCG干预组小鼠脾组织中Treg百分比(8.66%±0.48%)和地塞米松干预组小鼠脾组织中Treg百分比(5.54%±0.48%)均升高(P < 0.05);EGCG干预组小鼠脾组织中Treg百分比高于地塞米松组(P < 0.05)。见图 2。
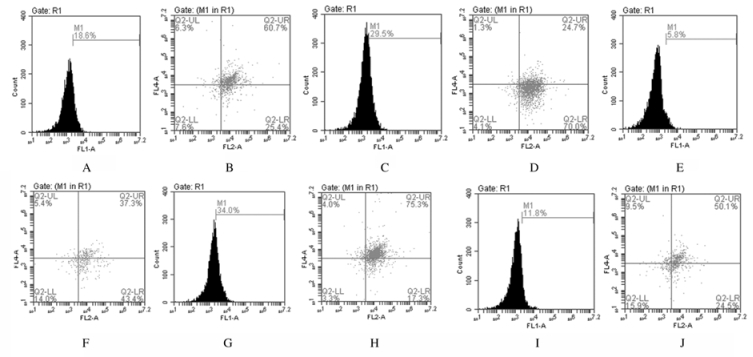
|
| 图 2 各组小鼠脾组织中Treg百分比 Fig. 2 Percentages of Treg in spleen tissue of mice in various groups |
|
|
与正常对照组(9.28%±0.74%)比较,肥胖组小鼠脾组织中Th17百分比(19.04%±0.66%)和肥胖型哮喘组小鼠脾组织中Th17百分比(37.98%±1.72%)均升高(P < 0.05);肥胖型哮喘组小鼠脾组织中Th17百分比高于肥胖组(P < 0.05)。与肥胖型哮喘组比较,EGCG干预组小鼠脾组织中Th17百分比(24.33%±1.52%)和地塞米松干预组小鼠脾组织中Th17百分比(25.60%±0.96%)均降低(P < 0.05);EGCG干预组小鼠脾组织中Th17百分比与地塞米松组比较差异无统计学意义(P>0.05)。见图 3。
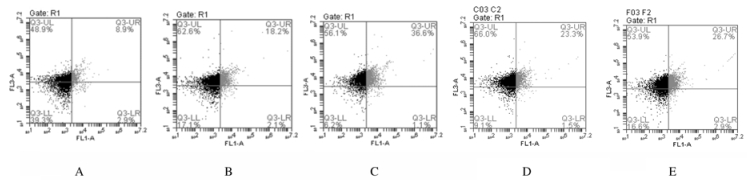
|
| 图 3 各组小鼠脾组织中Th17百分比 Fig. 3 Percentages of Th17 in spleen tissue of mice in various groups |
|
|
研究[14-15]显示:在高脂饮食诱导小鼠肥胖时,如肥胖鼠体质量大于正常鼠45%以上,肥胖鼠的气道反应性会明显升高。本研究中肥胖鼠的体质量均大于正常鼠平均体质量的50%以上。本研究结果表明:高脂饮食诱导肥胖小鼠气道反应性增高,而在此基础上进行OVA诱导和激发,肥胖型哮喘小鼠的气道反应性升高则更加明显;在给予EGCG干预后,肥胖型哮喘组小鼠的气道反应性降低,但给予地塞米松干预却不能降低气道反应性。上述结果与此前PARK等[16]的研究结论一致,地塞米松能够抑制肥胖型哮喘鼠的气道炎症,但不能缓解肥胖型哮喘引发的气道高反应性。这可能是因为肥胖会导致气道周围纤维化改变,而地塞米松对这种肥胖引发的气道周围纤维化无明显抑制作用。
本研究结果显示:高脂饮食诱导的肥胖小鼠血清中脂联素水平降低、瘦素水平升高,地塞米松会进一步降低脂联素水平、升高瘦素水平;EGCG干预能够抑制肥胖型哮喘小鼠瘦素水平的升高。此前有研究[12]显示给予EGCG干预能够明显抑制高脂饲料诱导的肥胖小鼠体质量增长,本研究结果与之不一致可能与该研究应用EGCG干预治疗的时间更长有关。
SANCHEZ-ZAUCO等[17]和LEIRIA等[18]的研究表明:肥胖型哮喘与经典哮喘的发病机制不同,其发生与Th2依赖的炎性进程无关,呈现Th1极化,即免疫应答向Th1方向过度发展。SCOTT等[19]对68例肥胖和47例非肥胖哮喘患者进行诱导痰细胞学分析后发现:肥胖与哮喘患者诱导痰中中性粒细胞数增多有关,虽然嗜酸细胞数在哮喘患者中也有所增加,但是其与肥胖无关。TELENGA等[20]的研究显示:肥胖型哮喘患者的血液和诱导痰中中性粒细胞数增加,因此推测肥胖可能导致中性粒细胞的浸润,肥胖型哮喘可能为中性粒细胞性哮喘。Treg/Th17失衡与中性粒细胞哮喘发生有密切关联,过度极化Th17能够分泌产生大量IL-17A,有效地动员、募集及活化中性粒细胞,从而介导嗜中性粒细胞炎症反应的发生。
本研究结果证实:在高脂饮食诱导的肥胖型哮喘小鼠中存在Treg/Th17免疫失衡,小鼠脾组织的CD4+T淋巴细胞中Th17百分比升高,BALF中Th17的效应细胞因子IL-17A水平升高;小鼠脾组织的CD4+T淋巴细胞中Treg百分比降低,BALF中Treg分泌的细胞因子IL-10水平降低。在高脂饮食诱导及OVA致敏、活化的肥胖型哮喘小鼠中,这种Treg/Th17免疫失衡则更为明显。
MATHEWS等[21]的研究显示:采用高脂肪饮食喂养的小鼠会出现气道反应性增高,而这种气道反应性的变化与Th17百分比及IL-17A水平有关,因Th17产生的IL-17A可直接作用于气道平滑肌促进气道高反应性。Th17及IL-17A在OVA诱导的哮喘小鼠中具有诱导气道炎症的作用[22],而抑制IL-17相关通路可以改善哮喘的气道炎症[23]。EGCG是绿茶中生物活性最强的儿茶素,相关研究[11, 24-25]显示:EGCG具有抗嗜酸性粒细胞性哮喘、抑制哮喘小鼠气道重塑发生的作用。本研究结果显示:EGCG干预治疗能够降低肥胖型哮喘小鼠BALF中异常升高的IL-17A水平和脾组织中Th17百分比,抑制过度的Th17免疫反应,从而发挥抑制肥胖型哮喘气道炎症及气道高反应性的作用。同时本研究结果也证实:EGCG能够提高肥胖型哮喘小鼠BALF中异常降低的IL-10水平和脾组织中Treg百分比,进一步帮助恢复Treg/Th17免疫失衡。
综上所述,在肥胖和肥胖型哮喘小鼠中均存在Treg/Th17免疫失衡;EGCG能够抑制肥胖型哮喘小鼠的气道炎症及气道高反应性,同时缓解肥胖型哮喘相关的Treg/Th17免疫失衡。本研究结果为EGCG治疗肥胖型哮喘提供了依据,绿茶提取物EGCG可能成为治疗肥胖型哮喘的有效药物。
| [1] | MONIKO M, PAWLICZAKR. Obesity and asthma:risk, control and treatment[J]. Postepy Dermatol Alergol, 2018, 35(6): 563–571. DOI:10.5114/ada.2018.77607 |
| [2] | Global strategy for asthma management and prevention. 2014 Global Initiative for Asthma[R/OL]. http://www.ginasthma.Org/.2014.8.12. |
| [3] | SASAKI M, YOSHIDA K, ADACHI Y, et al. Factors associated with asthma control in children:findings from a national Web-based survey[J]. Pediatr Allergy Immunol, 2014, 25(8): 804–809. |
| [4] | ZHAO J, LLOYD CM, NOBLE A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T cell-mediated tolerance and contribute to airway remodeling[J]. Mucosal Immunol, 2013, 6(2): 335–346. DOI:10.1038/mi.2012.76 |
| [5] | JIANG H, WU XB, ZHU HY, et al. FOXP3(+)Treg/Th17 cell imbalance in lung tissues of mice with asthma[J]. Int J Clin Exp Med, 2015, 8(3): 4158–4163. |
| [6] | JOEL AM, ALLISON PW, LUIZA R, et al. Induction of IL-17A precedes development of airway hyperresponsiveness during diet-induced obesity and correlates with complement factor D[J]. Front Immunol, 2014, 9(5): 440. |
| [7] | METIN D, ERKUT K, BURCU O, et al. CD4+, CD25+, FOXP3+ T regulatory cell levels in obese, asthmatic, asthmatic obese, and healthy children[J]. Inflammation, 2015, 38(4): 1473–1478. DOI:10.1007/s10753-015-0122-4 |
| [8] | GUPTA R K, GUPTA K, DWIVEDI P D. Pathophysiology of IL-33 and IL-17 in allergic disorders[J]. Cytokine Growth Factor Rev, 2017, 38: 22–36. DOI:10.1016/j.cytogfr.2017.09.005 |
| [9] | ORDOÑEZ C L, SHAUGHNESSY T E, MATTHAY M A, et al. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma:Clinical and biologic significance[J]. Am J Respir Crit Care Med, 2000, 161(4 Pt 1): 1185–1190. |
| [10] | ITO K, HERBERT C, SIEGLE J S, et al. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma[J]. Am J Respir Cell Mol Biol, 2008, 39(5): 543–550. DOI:10.1165/rcmb.2008-0028OC |
| [11] | YANG N, ZHANG H, CAI XX, et al. Epigallocatechin-3-gallate inhibits inflammation and epithelial-mesenchymal transition through the PI3K/AKT pathway via upregulation of PTEN in asthma[J]. Int J Mol Med, 2018, 41(2): 818–828. |
| [12] | BYUN J K, YOON B Y, JHUNA J Y, et al. Epigallocatechin-3-gallate ameliorates both obesity andautoinflammatory arthritis aggravated by obesity by altering thebalance among CD4+T-cell subsets[J]. Immunol Lett, 2014, 157(1/2): 51–59. |
| [13] | HENDERSON W RJR, TANG L O, CHU S J, et al. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model[J]. Am J Respir Crit Care Med, 2002, 165(1): 108–116. DOI:10.1164/ajrccm.165.1.2105051 |
| [14] | KIM H Y, LEE H J, CHANG Y J, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity[J]. Nat Med, 2014, 20(1): 54–61. DOI:10.1038/nm.3423 |
| [15] | JUNG S H, KWON J M, SHIM J W, et al. Effects of diet-induced mild obesity on airway hyperreactivity and lung inflammation in mice[J]. Yonsei Med J, 2013, 54(6): 1430–1437. DOI:10.3349/ymj.2013.54.6.1430 |
| [16] | PARK H J, LEE J H, PARK Y H, et al. Roflumilast ameliorates airway hyperresponsiveness caused by diet-induced obesity in a murine model[J]. Am J Respir Cell Mol Biol, 2016, 55(1): 82–91. DOI:10.1165/rcmb.2015-0345OC |
| [17] | SÁNCHEZ-ZAUCO N, DEL RIO-NAVARRO B, GALLARDO-CASAS C, et al. High expression of Toll-like receptors 2 and 9 and Th1/Th2 cytokines profile in obese asthmatic children[J]. Allergy Asthma Proc, 2014, 35(3): 34–41. |
| [18] | LEIRIA L O, MARTINS M A, SAAD M J. Obesity and asthma:beyond T(H)2 inflammation[J]. Metab Clin Exp, 2015, 64(2): 172–181. DOI:10.1016/j.metabol.2014.10.002 |
| [19] | SCOTT H A, GIBSON P G, GARG M L, et al. Airway inflammation is augmented by obesity and fatty acids in asthma[J]. Eur Respir J, 2011, 38(3): 594–602. DOI:10.1183/09031936.00139810 |
| [20] | TELENGA E D, TIDEMAN S W, KERSTJENS H A, et al. Obesity in asthma:more neutrophilic inflammation as a possible explanation for a reduced treatment response[J]. Allergy, 2012, 67(8): 1060–1068. DOI:10.1111/j.1398-9995.2012.02855.x |
| [21] | MATHEWS J A, WURMBRAND A P, NETO F L, et al. Induction of IL-17A precedes development of airway hyperresponsiveness during diet induced obesity and correlates with complement factor D[J]. Front Immunol, 2014, 15(5): 440. |
| [22] | KANELLOPOULOU C, MULJO S A. Fine-tuning Th17 cells:to be or not to be pathogenic[J]. Immunity, 2016, 44(6): 1241–1243. DOI:10.1016/j.immuni.2016.06.003 |
| [23] | ZHANG W, ZHANG X, SHENG A, et al. γ-Secretase gamma-secretase inhibitor alleviates acute airway inflammation of allergic asthma in mice by downregulating Th17 cell differentiation[J]. Mediators Inflam, 2015: 258168. |
| [24] | LISHEN D, XINYUAN K, FEN L, et al. Epigallocatechin gallate improves airway inflammation through TGF-β1 signaling pathway in asthmatic mice[J]. Mol Med Rep, 2018, 18(2): 2088–2096. |
| [25] | CHOI Y S, BAE C H, SONG S Y, et al. The effect of Epigallocatechin-3-gallate in allergic airway inflammation[J]. Rhinology, 2014, 52(4): 406–412. DOI:10.4193/Rhin |
 2019, Vol. 45
2019, Vol. 45


