扩展功能
文章信息
- 赵一安, 杨舒婷, 李亚龙, 刘国民, 孙淑芬, 罗云纲
- ZHAO Yian, YANG Shuting, LI Yalong, LIU Guomin, SUN Shufen, LUO Yungang
- 静电喷雾法制备聚乳酸-羟基乙酸共聚物/羟基磷灰石微载体的工艺及其效果评价
- Preparation process of polylactic acid-glycolic acid copolymer/hydroxyapatite microcarrier by electrostatic spraying method and its effect evaluation
- 吉林大学学报(医学版), 2019, 45(02): 439-444
- Journal of Jilin University (Medicine Edition), 2019, 45(02): 439-444
- 10.13481/j.1671-587x.20190241
-
文章历史
- 收稿日期: 2018-06-25
2. 吉林大学口腔医院修复科, 吉林 长春 130021;
3. 吉林大学第二医院骨科, 吉林 长春 130041;
4. 吉林大学口腔医院牙体牙髓科, 吉林 长春 130021
2. Department of Prosthodontics, Stomatology Hospital, Jilin University, Changchun 130021, China;
3. Department of Orthopedics, Second Hospital, Jilin University, Changchun 130041, China;
4. Department of Endodontics and Operative Dentistry, Stomatology Hospital, Jilin University, Changchun 130021, China
牙周疾病治疗后牙周组织的完全再生是一个尚未解决的问题[1]。目前临床所应用的牙周组织再生术中使用的骨组织支架材料大多有其局限性,如支架材料具有抗原性、降解速率难以调控和强度较差等,并不能完全满足临床需要[2-3]。以细胞微载体为骨组织工程支架材料是目前有关研究的重要方向[4]。微载体培养能够加速牙周膜基质细胞的增殖,使成骨相关基因表达上调和牙骨质蛋白23、骨涎蛋白、骨桥蛋白及骨膜素上调[5]。有研究[6]通过运用聚乳酸-羟基乙酸共聚物微球顺序释放骨生长肽(osteogenic growth peptide, OGP)和骨形态发生蛋白2(bone morphogenetic protein-2,BMP-2)模式来促进骨髓间充质干细胞(mesnchymal stem cells, BMSCs)的成骨分化。聚乳酸-羟基乙酸共聚物(polylactic-co-glycolic acid, PLGA)因其良好的生物相容性与降解性能可作为原料制备微载体,并取得较多研究进展[7]。但PLGA缺乏天然识别位点,既不能促进细胞附着,也不具备骨诱导能力[8]。羟基磷灰石(hydroxyapatite, HA)与人体骨组织结构相近,且具有优良的组织相容性和生物传导性,是理想的骨缺损修复材料[9],但其降解性能较差[10]。复合PLGA/HA体系可同时获得二者的优势性能,构建出兼备成骨活性与降解性能的载体材料。静电喷雾技术是一种利用电流体动力学原理将聚合物溶液或溶体制成微、纳米级粒子或纤维物质的方法[11]。沈文等[12]利用静电喷雾法制备聚乳酸(polylactic acid,PLA)负载阿维菌素(avermectin, AVM)的载药微球,粒径可达5~20 μm。本实验利用静电喷雾技术,通过调整电压、接受距离和推进速度等参数制备微载体。利用静电发生器在喷头与接收液之间形成高压静电场,工作液流经喷头时被带上电荷,在静电力与重力的作用下,液滴做定向运动,最终在接收液中固定。可以通过调节工作液的推进速度、喷头距离接收液面的高度和电压等控制微载体的形态、尺寸。该方法制备的微载体颗粒均匀一致、结构形态可控,还可避免传统方法如乳液溶剂挥发法[13]和喷雾干燥法[14]等大量使用有机溶剂和乳剂并难以去除及应用局限等问题,在药物缓释和骨组织工程支架等领域具有广泛的应用前景。本实验应用高压静电喷雾技术,以PLGA/HA为工作液、60%乙醇为接收液制备微载体,并观察该方法制备的微载体的特征,为细胞微载体构建提供参考。
1 材料与方法 1.1 主要试剂和仪器N-甲基吡咯烷酮(NMPA,AR>99.0%)(上海阿拉丁生化科技股份有限公司),DMEM培养基(美国Hyclone公司),FBS血清(美国Gibco公司), PLGA(LA/GA=50/50,长春圣博玛生物材料有限公司),HA(20 nm,北京德科岛金科技有限公司), 无水乙醇(北京化工厂)。电子天平(福州华志科学仪器有限公司),磁力搅拌器和恒温干燥箱(上海精宏实验设备有限公司),静电发生器(上海孚蕊哲静电科技有限公司),CKX41SF型激光共聚焦显微镜(laser scanning confocal microscope, LSCM)(日本Olympus公司)。
1.2 PLGA/HA基液的配制称取0.5 g PLGA混入10 mL NMPA溶剂中,配制质量浓度为5%的PLGA溶液,70℃水浴条件下,磁力搅拌12 h,加入质量浓度为3%的HA粉末,70℃水浴条件下,磁力搅拌12h,再次混匀。
1.3 PLGA/HA微载体制备连接静电发生器与自动推进装置(图 1),将接收液置于喷头正下方,喷头距离接收液面12 cm,用5 mL注射器抽取配制好的工作液置于推进器中,选择27G针头(D=0.15 mm),调节参数,推进速度0.47 m·h-1, 电压4.5 kV,喷头距离接收液面12 cm,接入自动搅拌装置,制备同时搅拌接收液,使微载体体系分散。

|
| 图 1 静电喷雾法装置示意图 Fig. 1 Diagram of electrospinning device |
|
|
扫描电镜(scanning electron microscope, SEM)观察静电喷雾法制备的3% HA浓度的PLGA/HA复合微载体的表面形态, 鬼笔环肽(FITC Phalloidin)和4’, 6-二脒基-2-苯基吲哚(4’, 6-diamidino-2-phenylindole,DAPI)染色观察微载体表面MC3T3-E1细胞分布情况,傅里叶变换红外光谱仪(fourier transform infrared spectroscope, FTIR)对微载体进行定性分析。
1.5 MC3T3-E1细胞复苏和培养取出-80℃冻存的MC3T3-E1细胞,快速置于37℃水浴,并不断摇动,使液体快速融化,1 min后取出,加入5 mL完全培养基,吹打均匀后,1500r·min-1离心5 min,吸除上清液,将细胞沉淀吹入完全培养基,于5%CO2、37℃培养箱中孵育,至细胞融合达80%。
1.6 细胞增殖实验检测微载体细胞亲和性和细胞毒性以PLGA微载体为PLGA组,PGLA+1%HA为1%PGLA/HA组,PGLA+3%HA为3%PGLA/HA组, PGLA+5%HA为5%PGLA/HA组,单纯细胞为空白对照组。将各组微载体与MC3T3-E1细胞共培养。取各组适量微载体平铺覆盖12孔板板底70%~80%,每孔3复孔,并加入2×104个MC3T3-E1细胞,于培养箱中孵育,72 h后首次换液,之后每隔48 h换液1次。共培养4和7d后吸弃上清,PBS冲洗3次,加入无血清培养基和CCK-8溶液,培养箱孵育2h,吸取上清,转移至96孔板,酶标仪检测吸光度(A)值,A值与活细胞数量成正比,以A值代表细胞数量,取平均值,绘制柱状图。
1.7 荧光染色观察MC3T3-E1细胞在PLGA/HA微载体上的生长和分布将各组微载体与MC3T3-E1细胞共培养至细胞融合80%,去除培养基,PBS冲洗,加入4%多聚甲醛固定10 min,再行PBS冲洗3次,分别进行FITC和4’, 6-DAPI染色,PBS冲洗3次,染色过程注意避光,LSCM观察MC3T3-E1细胞在PLGA/HA微载体上的生长状态和分布情况。
1.8 统计学分析采用GraphPad Prism7统计软件进行统计学分析。各组细胞增殖实验中A值以x±s表示,多组间样本均数比较采用单因素方差分析。以P<0.05为差异有统计学意义。
2 结果 2.1 不同电压下微载体形貌电压在4.0~4.5 kV范围内微载体形态趋于稳定,呈球形,粒径在4.5 kV时趋于稳定,电压大于5 kV时微球形态不规则。见表 1。
| HA (η/%) |
Voltage (V/kV) |
Particle size (d/μm) |
Microsphere appearance |
| 3 | 4.0 | 200-350 | Sphere |
| 3 | 4.5 | 350-380 | Sphere |
| 3 | >5.0 | 50-400 | Irregular sphere/ Hemisphere |
倒置显微镜下见微载体边缘光滑,大小形态一致,微载体之间无粘连,各组微载体形态表现未见差异。见图 2。

|
| A:1%PLGA/HA group; B:3%PLGA/HA group; C:5%PLGA/HA group. 图 2 倒置显微镜观察各组PLGA/HA微载体形态表现(Bar=200 μm) Fig. 2 Morphology of PLGA/HA microcarriers in various groups detected by inverted microscope(Bar=200 μm) |
|
|
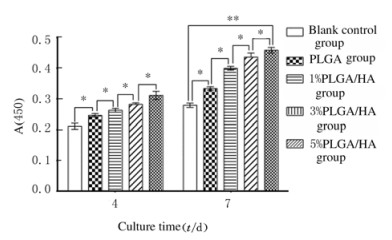
PLGA组、1%PLGA/HA组、3%PLGA/HA组和5%PLGA/HA组细胞数量均高于空白对照组(P < 0.05), 随着HA质量浓度升高,微载体对细胞的促进增殖作用越明显。在培养7d时,5%PLGA/HA组细胞数量明显高于PLGA组(P < 0.01)。见图 3。
|
| 图 3 各组细胞增殖实验结果 Fig. 3 Cell proliferation test results in various groups |
|
|
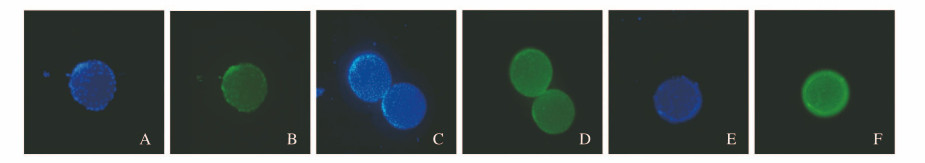
FITC与DAPI染色后细胞在PLGA/HA微载体上的生长状态与分布情况:蓝色荧光为DAPI染色细胞核,绿色荧光为FITC染色肌动蛋白微丝,MC3T3-E1细胞生长状态良好,均匀黏附分布在PLGA/HA微载体表面。3%PLGA/HA组和5%PLGA/HA组被染色细胞数量均高于1%PLGA/HA组。见图 4(插页五)。
|
| A, B:1%PLGA/HA; CD:3%PLGA/HA; E, F:5%PLGA/HA; A, C, E:DAPI staining; BD, F:FITC staining. 图 4 LSCM观察各组MC3T3-E1细胞的生长(Bar=200 μm) Fig. 4 Growth of MC3T3-E1 cells in various groups observed by LSCM(Bar=200 μm) |
|
|
SEM观察结果:微载体颗粒均匀,均为椭圆形或圆形,无异常形态球,表面光滑,无锐利边缘,球体之间无粘连、无聚集,各组微载体表面形态之间无明显差别。见图 5。

|
| A, B:1%PLGA/HA group; C, D:3%PLGA/HA group; E, F:5%PLGA/HA group; A, C, E:Bar=500 μm; B, D, F:Bar=100 μm. 图 5 SEM观察各组PLGA/HA微载体表面形态 Fig. 5 Surface morphology of PLGA/HA microcarriers in various groups observed by SEM |
|
|
PLGA特征吸收位于2 990-1与2 940 cm-1(-CH3),1753 cm-1(C=O),1183与1083 cm-1(C-O)。在500~600 cm-1(P-O)处观察到HA特征吸收峰,且吸收峰高度5%PLGA/HA组>3%PLGA/HA组>1%PLGA/HA组, 表明HA混入PLGA溶液中, 制得PLGA/HA复合微载体。见图 6。

|
| The arrow denoted the characteristic absorption peak of HA. 图 6 各组PLGA/HA微载体FTIR分析结果 Fig. 6 FTIR analysis result of PLGA/HA microcarriers in various groups |
|
|
PLGA的优异性能是其生物降解性可以通过改变乳酸(lactic acid, LA)和乙醇酸(glycolic acid, GA)的比例来控制[15],被广泛应用于医疗领域。微球的形状、直径、粒度分布、表面形貌和化学成分等可能影响细胞的黏附和增殖,对于微球在组织工程中的成功应用至关重要[16-17]。纳米HA在骨组织工程中的应用日渐成熟。研究[18]显示:PLGA和HA纳米复合材料在支架形式中有优异的生物降解性,而PLGA/HA微载体在体内也具有相似的生物降解性[19]。本实验采用静电喷雾技术制备PLGA/HA微载体,优化微载体制备工艺,相对于乳液溶剂挥发法和相分离法其操作更加简单便捷。该方法在高出球率的前提下获得表面均匀、大小一致的细胞微载体,对细胞微载体的制备工艺进行了改良。本研究结果显示:PLGA/HA微载体表面细胞生长及黏附状态良好,能够有效地支持细胞的黏附与扩增,具有细胞亲和力,无细胞毒性。PLGA/HA微载体相对于PLGA微载体对细胞的增殖促进作用更强,ZHANG等[20]在研究中也证实了PLGA/HA支架相对于PLGA支架有更优异的生物性能;随着HA浓度的增加。细胞增殖量也随之增加。HE等[21]也证实高浓度HA表现出比低浓度HA复合支架更好的生物相容性及骨形成,为后续研究更高HA浓度的微载体对细胞的增殖促进作用提供了研究思路。
与乳化溶剂挥发法和相分离法等比较,本实验应用静电喷雾法制备的微载体具有更多优势性能。陈红等[22]在对比实验中得出结论:乳化溶剂挥发法制得的微球分散性差,大小不均,粘连程度高。本实验所制得微载体分散、无粘连,且颗粒均匀一致。乳化溶剂挥发法制得的微球不利于细胞黏附,油相与水相比也会影响微球的粒径[23]。本实验证实静电喷雾法制得微球粒径均匀一致,细胞黏附状态良好。喷雾干燥法在高温条件下制球,在材料选择上有较多局限,而且不利于加入生物活性物质[24]。静电喷雾法制球条件温和,适于添加生物活性物质,制球材料选择更为广泛。
综上所述,本实验成功运用静电喷雾法制得PLGA/HA微载体并进行测试,可作为后续实施体内实验的基础。本研究结果为细胞微载体相关研究与制备方法提供参考,该种微载体在骨组织工程支架材料领域具有广泛应用潜力。
| [1] | SIAILI M, CHATZOPOULOU D, GILLAM D G. An overview of periodontal regenerative procedures for the general dental practitioner[J]. Saudi Dent J, 2018, 30(1): 26–37. DOI:10.1016/j.sdentj.2017.11.001 |
| [2] | YADEGARI A, FAHIMIPOUR F, RASOULIANBOROUJENI M, et al. Specific considerations in scaffold design for oral tissue engineering[J]. Biomater Oral Dent Tissue Engineer, 2017, 10: 157–183. |
| [3] | VENKATESAN J, KIM S K. Nano-hydroxyapatite composite biomaterials for bone tissue engineering-a review[J]. J Biomed Nanotechnol, 2014, 10(10): 3124–3140. DOI:10.1166/jbn.2014.1893 |
| [4] | LEE Y S, LIM K S, OH J E, et al. Development of porous PLGA/PEI1.8k biodegradable microspheres for the delivery of mesenchymal stem cells (MSCs)[J]. J Controll Release, 2015, 205: 128–133. DOI:10.1016/j.jconrel.2015.01.004 |
| [5] | CEBATARIUNIENÉ A, JARMALAVICIUTÉ A, TUNAITIS V, et al. Microcarrier culture enhances osteogenic potential of human periodontal ligament stromal cells[J]. J Cranio-maxillo-facial Surg, 2017, 45(6): 845–854. DOI:10.1016/j.jcms.2017.03.009 |
| [6] | ZHANG B, HAN Z, DUAN K, et al. Multilayered pore-closed PLGA microsphere delivering OGP and BMP-2 in sequential release patterns for the facilitation of BMSCs osteogenic differentiation[J]. J Biomed Mater Res Part A, 2017, 106(1): 95–105. |
| [7] | SUN X, XU C, WU G, et al. Poly(Lactic-co-glycolic acid):Applications and future prospects for periodontal tissue regeneration[J]. Polymers, 2017, 9(6): 189–207. |
| [8] | 马骊娜, 方大为, 王克敏, 等. 静电喷雾法制备壳聚糖/康普瑞丁载药微载体[J]. 材料科学与工程学报, 2015, 33(6): 889–894. |
| [9] | 龙海波, 刘剑军, 熊龙. 载rhBMP-2中空HA微载体修复骨缺损实验研究[J]. 江西医药, 2016, 51(3): 238–243. DOI:10.3969/j.issn.1006-2238.2016.03.016 |
| [10] | VAN LANDUYT P, LI F, KEUSTERMANS J P, et al. The influence of high sintering temperatures on themechanical properties of hydroxyapatite[J]. J Mater Sci Mater Med, 1995, 6: 8–13. DOI:10.1007/BF00121239 |
| [11] | ABHIJIT P, SHREYA T, MANJU M. A bird's eye view of nanoparticles prepared by electrospraying:advancements in drug delivery field[J]. J Control Release, 2018, 286: 179–200. DOI:10.1016/j.jconrel.2018.07.036 |
| [12] | 沈文, 张光华, 李亚莉, 等. 静电喷雾法制备AVM/PLA载药微载体及光降解性能的研究[J]. 应用化工, 2017, 46(11): 2170–2173. DOI:10.3969/j.issn.1671-3206.2017.11.025 |
| [13] | SHI W C, DIDIER J E, INGBER D E, et al. Collective shape actuation of polymer double emulsions by solvent evaporation[J]. ACS Appl Mater Interfaces, 2018, 10(38): 31865–31869. DOI:10.1021/acsami.8b13216 |
| [14] | IRE M. Microencapsulation by spray drying[J]. Drying Technol, 1998, 16(6): 1195–1236. DOI:10.1080/07373939808917460 |
| [15] | WANG J, WANG L, ZHOU Z, et al. Biodegradable polymer membranes applied in guided bone/tissue regeneration:A review[J]. Polymers, 2016, 8(4): 115–135. |
| [16] | BERTOLO A, HÄFNER S, TADDEI A R, et al. Injectable microcarriers as human mesenchymal stem cell support and their application for cartilage and degenerated intervertebral disc repair[J]. Eur Cell Mater, 2015, 29: 70–81. DOI:10.22203/eCM |
| [17] | THISSEN H, CHANG K Y, TEBB T A, et al. Synthetic biodegradable microparticles for articular cartilage tissue engineering[J]. J Biomed Mater Res Part A, 2006, 77(3): 590–598. |
| [18] | TAINIO J, PAAKINAHO K, AHOLA N, et al. In vitro degradation of borosilicate bioactive glass and poly(l-lactide-co-ε-caprolactone) composite scaffolds[J]. Materials(Basel), 2017, 10(11): E1274. |
| [19] | GAO T L, ZHANG N, WANG Z L, et al. Biodegradable microcarriers of poly (lactide-co-glycolide) and nano-hydroxyapatite decorated with IGF-1 via polydopamine coating for enhancing cell proliferation and osteogenic differentiation[J]. Macromol Biosci, 2015, 15(8): 1070–1080. DOI:10.1002/mabi.v15.8 |
| [20] | ZHANG B, ZHANG P B, WANG Z L, et al. Tissue-engineered composite scaffold of poly(lactide-co-glycolide) and hydroxyapatite nanoparticles seeded with autologous mesenchymal stem cells for bone regeneration[J]. J Zhejiang Univ Sci B, 2017, 18(11): 963–976. DOI:10.1631/jzus.B1600412 |
| [21] | HE S, LIN K F, SUN Z, et al. Effects of nano-hydroxyapatite/poly(dl-lactic-co-glycolic acid) microsphere-based composite scaffolds on repair of bone defects:evaluating the role of nano-hydroxyapatite content[J]. Artif Organs, 2016, 40(7): E128–E135. DOI:10.1111/aor.2016.40.issue-7 |
| [22] | 陈红, 徐菊美, 赵世成, 等. 微流控法制备PLGA微球及其性能研究[J]. 现代化工, 2018, 38(1): 129–132. |
| [23] | WANG H O, LI P, ZHANG L K, et al. Preparation and in vitro release characteristics of curcumin loaded biodegradable microspheres[J]. Zhongguo Zhongyao Zazhi, 2009, 34(23): 3021–3024. |
| [24] | SINGH S K, VUDDANDA P R, SINGH S, et al. A comparison between use of spray and freeze drying techniques for preparation of solid self-microemulsifying formulation of valsartan and in vitro and in vivo evaluation[J]. Biomed Res Int, 2013, 2013: 909045. |
 2019, Vol. 45
2019, Vol. 45


