扩展功能
文章信息
- 梅杰, 周文莉, 闫佳秀, 郭世杰
- MEI Jie, ZHOU Wenli, YAN Jiaxiu, GUO Shijie
- 小头畸形-癫痫-发育迟缓症1例报告及其遗传家系图谱分析
- A case report of microcephaly, seizures and developmental delay and analysis on its genetic pedigree
- 吉林大学学报(医学版), 2019, 45(02): 422-425
- Journal of Jilin University (Medicine Edition), 2019, 45(02): 422-425
- 10.13481/j.1671-587x.20190237
-
文章历史
- 收稿日期: 2018-11-02
小头畸形-癫痫-发育迟缓症(microcephaly, seizures and developmental delay,MCSZ)是一种近几年才明确的罕见的神经系统发育障碍疾病,全世界仅报道10余例[1-4],迄今无中国人群病例的相关报道。MCSZ临床表现为小头畸形、早发难治性癫痫和发育迟缓。该病为常染色体隐性遗传,由多核苷酸激酶/磷酸酶(polynucleotide kinase/phosphatase,PNKP)基因功能性突变所引起[1, 5]。本文作者报道1例MCSZ男性患儿,患儿临床表现为出生时前囟及头围小、新生儿期即出现难治性癫痫、发育迟缓,4个月时因持续抽搐发作死亡。该患儿PNKP基因突变为新发现且为首次报道,本文作者对MCSZ患儿的临床诊治进行分析,旨在提高临床医生对MCSZ的认识。
1 临床资料 1.1 一般资料先证者:男性,1.5 h,因母孕36周,生后1.5 h入院。系2胎2产,因羊水极少,产程未发动选择性剖宫产娩出,出生体质量2.1 kg,否认宫内窘迫及生后窒息史,胎盘、脐带无异常。病程中患儿无呼吸困难,无发热,未开奶,尿便未排。入院查体:一般状态及反应好,皮肤红润;早产儿外貌:皮肤光滑、中等厚度,指甲已达指尖,足底纹理褶痕>前2/3,乳晕呈点状,边缘不突起,直径 < 0.75 cm。前囟0.5 cm×0.5 cm,平坦、无紧张,头围29 cm(小于同胎龄儿第3百分位);呼吸56 min-1,三凹征阴性,双肺呼吸音清,未闻及啰音;心率112 min-1,心音有力,节律规整,心前区未闻及杂音;腹软,肝脾肋下未触及,肠鸣音正常;四肢暖,脉搏搏动正常,四肢肌张力及原始反射正常。患儿父母健康,非近亲结婚;患儿4岁哥哥健康。
1.2 实验室和辅助检查血常规、心肌酶、肝功、肾功和血气分析均正常。头部MRI平扫显示前脑无裂畸形,白质和小脑发育不良,枕大池增大,扁平颅底。见图 1。

|
| A:Holoprosencephaly and dysplasia of the white matter; B:Dysplasia of the cerebellum, enlargement of the cisterna magna and flat skull base. 图 1 MCSZ患儿头部MRI平扫图 Fig. 1 Brain MRI plain scan of MCSZ patient |
|
|
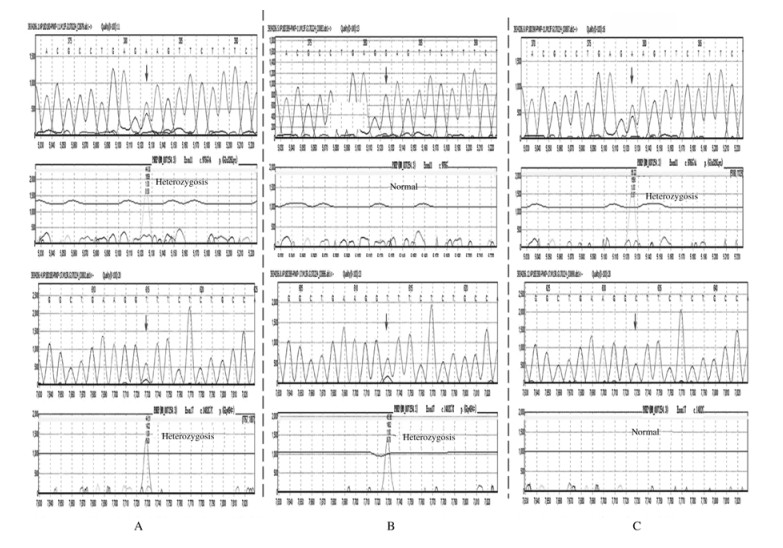
采集患儿及其父母的血样进行全外显子组基因测序,结果显示:患儿PNKP基因存在杂合致病突变c.976G > A (p.Glu326Lys)和临床意义未明的杂合突变c.1482C > T (p.Gly494=),患儿为复合杂合突变。其母携带相同杂合致病突变c.976G > A (p.Glu326Lys),其父携带相同临床意义未明的杂合突变c.1482C > T (p.Gly494=)。见图 2。
|
| A:Compound heterozygous mutations of c.976G>A (p.Glu326Lys) and c.1482C>T (p.Gly494=) in the proband (arrow); B:Heterozygous mutation of c.1482C>T (p.Gly494=) in the father of proband (arrow); C:Heterozygous mutation of c.976G>A (p.Glu326Lys) in the mother of proband (arrow). 图 2 MCSZ患儿及其父母的PNKP基因测序图 Fig. 2 Sequencing maps of PNKP genes of MCSZ patient and his parents |
|
|
患儿出生后第15天出现抽搐,表现为意识丧失、频繁眨眼、口周发绀和四肢抽动,每次持续数秒钟,每日发作10余次。脑电图见弥漫性阵发性尖波和棘波。给予抗癫痫治疗(托吡酯和左乙拉西坦),无效;患儿2个月睡眠时无自发微笑,逗之不会笑,眼睛无追视,3个月不能抬头。4个月时因持续抽搐发作死亡,临床诊断为MCSZ。
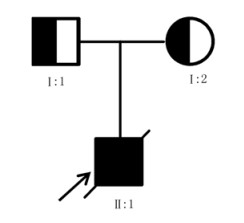
1.5 家系图谱依据全外显子组基因测序检测结果,考虑患儿存在的复合杂合突变,其中与母亲相同的杂合突变c.976G > A (p.Glu326Lys)为已经明确的致病杂合突变,而与父亲相同的杂合突变c.1482C > T(p.Gly494=)可能为新发现的致病杂合突变,绘制家系图谱。见图 3。
|
| 图 3 MCSZ患儿家系图谱 Fig. 3 Pedigree of MCSZ patient |
|
|
小头畸形是一类神经系统发育障碍性疾病,其主要临床特征为头围减小,由于纳入研究人群的种族及诊断标准不同,活产儿中小头畸形的发病率差异较大,其发病率为(1.3~150.0)/ 10万[6]。小头畸形既可单独存在,也可作为许多遗传性综合征的一部分表现,有些患者可通过鉴定致病基因明确病因[7-13]。MCSZ是一种伴有小头畸形的罕见的神经系统发育障碍综合征,临床特征包括小头畸形,早发的难治性癫痫、发育迟缓和行为问题,尤其是多动症。该例患儿以出生后头围明显减小为主要症状,头部MRI平扫见明显脑发育异常,表现为前脑无裂畸形,白质及小脑发育不良,枕大池增大,扁平颅底,通过全外显子组基因测序确定存在PNKP基因的复合杂合突变,结合患儿出生后第15天出现频繁抽搐,发育明显落后,明确临床诊断为MCSZ。给予抗癫痫治疗,但无效,出生后4个月因持续抽搐发作死亡。
MCSZ是由于PNKP基因突变所致,PNKP蛋白是一种同时具有DNA 5′-激酶和DNA3′-磷酸酶活性的参与DNA单链和双链断裂修复的关键酶,这一DNA修复过程在神经系统中尤为重要[5, 14-16]。单链DNA断裂可内源性发生在正常细胞代谢过程中,具有DNA毒性作用,损伤基因组完整性,由于PNKP既具有3′-磷酸激酶又具有5′-激酶的活性,因而具有终止单链断裂损伤的能力[17]。同样,在双链DNA断裂修复过程中,该酶可以同时作为非同源末端连接及碱基切除修复通路的功能部分,起到双链断裂修复的作用。由于在神经元细胞中的高转录需求以及神经系统暴露在高水平氧化应激下,极易发生断裂损伤,而这些DNA单双链断裂即可特异性地影响神经元[18]。DNA修复障碍可加速的细胞死亡,导致神经系统发育不良和神经变性。PNKP基因突变除导致MCSZ外,还可导致进行性小脑萎缩和多发性神经病[19]、共济失调伴眼动失用症4型[20]。PNKP突变导致不同的神经疾病的根本原因仍然不确定,但是这可能反映了不同突变对PNKP功能的影响和(或)该蛋白在非同源末端连接中的其他作用。
目前文献[1]报道:MCSZ患者具有一定程度表型异质性,表型可以从无任何脑萎缩改变或者临床神经系统退行性变到明显的神经系统病变,如进行性小脑萎缩和感觉运动性周围神经病变。癫痫表型也各不相同,可表现为严重的早发婴儿癫痫性脑病,而进行性神经元退行性变的患者则表现为相对较轻的癫痫形式,偶有癫痫发作,并可随年龄增长有所改善。该例患儿为典型的MCSZ,新生儿期即表现出严重的癫痫发作,且抗癫痫治疗无效。最终因持续抽搐发作死亡。患儿的父母往往均携带致病突变,携带致病突变的父母每次生育子女均有25%的可能患有该病;患者父母的其他亲属亦具有携带相同致病突变的风险,因此临床一旦发现可疑病例应积极行相关基因检测,明确诊断后应建议患者父母再孕时进行遗传咨询,降低MCSZ再发风险,提高活产儿质量。
| [1] | SHEN J, GILMORE E C, MARSHALL C A, et al. Mutations in PNKP cause microcephaly, seizures and defects in DNA repair[J]. Nat Genet, 2010, 42(3): 245–249. DOI:10.1038/ng.526 |
| [2] | NAKASHIMA M, TAKANO K, OSAKA H, et al. Causative novel PNKP mutations and concomitant PCDH15 mutations in a patient with microcephaly with early-onset seizures and developmental delay syndrome and hearing loss[J]. J Hum Genet, 2014, 59(8): 471–474. DOI:10.1038/jhg.2014.51 |
| [3] | NAIR P, HAMZEH A R, MOHAMED M, et al. Microcephalic primordial dwarfism in an Emirati patient with PNKP mutation[J]. Am J Med Genet A, 2016, 170(8): 2127–2132. DOI:10.1002/ajmg.a.v170.8 |
| [4] | ENTEZAM M, RAZIPOUR M, TALEBI S, et al. Multi affected pedigree with congenital microcephaly:WES revealed PNKP gene mutation[J]. Brain Dev, 2018. DOI:10.1016/j.braindev.2018.08.005 |
| [5] | REYNOLDS J J, WALKER A K, GILMORE E C, et al. Impact of PNKP mutations associated with microcephaly, seizures and developmental delay on enzyme activity and DNA strand break repair[J]. Nucleic Acids Res, 2012, 40(14): 6608–6619. DOI:10.1093/nar/gks318 |
| [6] | HASHMI J A, AL-HARBI K M, RAMZAN K, et al. A novel splice-site mutation in the ASPM gene underlies autosomal recessive primary microcephaly[J]. Ann Saudi Med, 2016, 36(6): 391–396. DOI:10.5144/0256-4947.2016.391 |
| [7] | YE Y Z, CHO M T, RETTERER K, et al. De novo POGZ mutations are associated with neurodevelopmental disorders and microcephaly[J]. Cold Spring Harb Mol Case Stud, 2015, 1(1): a000455. DOI:10.1101/mcs.a000455 |
| [8] | FAHEEM M, NASEER M I, RASOOL M, et al. Molecular genetics of human primary microcephaly:an overview[J]. BMC Med Genomics, 2015, 8(Suppl 1): S4. |
| [9] | DOOBIN D J, KEMAL S, DANTAS T J, et al. Severe NDE1-mediated microcephaly results from neural progenitor cell cycle arrests at multiple specific stages[J]. Nat Commun, 2016, 7: 12551. DOI:10.1038/ncomms12551 |
| [10] | RUMP P, JAZAYERI O, van DIJK-BOS K K, et al. Whole-exome sequencing is a powerful approach for establishing the etiological diagnosis in patients with intellectual disability and microcephaly[J]. BMC Med Genomics, 2016, 9: 7. |
| [11] | BASIT S, AL-HARBI K M, ALHIJJI S A, et al. CIT, a gene involved in neurogenic cytokinesis, is mutated in human primary microcephaly[J]. Hum Genet, 2016, 135(10): 1199–1207. DOI:10.1007/s00439-016-1724-0 |
| [12] | HANZLIK E, GIGANTE J. Microcephaly[J]. Children, 2017, 4(6): E47. DOI:10.3390/children4060047 |
| [13] | KAYMAKCALAN H, YARMAN Y, GOC N, et al. Novel compound heterozygous mutations in GPT2 linked to microcephaly, and intellectual developmental disability with or without spastic paraplegia[J]. Am J Med Genet A, 2018, 176(2): 421–425. DOI:10.1002/ajmg.a.38558 |
| [14] | WEINFELD M, MANI R S, ABDOU I, et al. Tidying up loose ends:the role of polynucleotide kinase/phosphatase in DNA strand break repair[J]. Trends Biochem Sci, 2011, 36(5): 262–271. DOI:10.1016/j.tibs.2011.01.006 |
| [15] | IYAMA T, WILSON D M 3rd. DNA repair mechanisms in dividing and non-dividing cells[J]. DNA Repair (Amst), 2013, 12(8): 620–636. DOI:10.1016/j.dnarep.2013.04.015 |
| [16] | SHIMADA M, DUMITRACHE L C, RUSSELL H R, et al. Polynucleotide kinase-phosphatase enables neurogenesis via multiple DNA repair pathways to maintain genome stability[J]. EMBO J, 2015, 34(19): 2465–2480. DOI:10.15252/embj.201591363 |
| [17] | BRESLIN C, MANI R S, FANTA M, et al. The Rev1 interacting region (RIR) motif in the scaffold protein XRCC1 mediates a low-affinity interaction with polynucleotide kinase/phosphatase (PNKP) during DNA single-strand break repair[J]. J Biol Chem, 2017, 292(39): 16024–16031. DOI:10.1074/jbc.M117.806638 |
| [18] | REYNOLDS J J, STEWART G S. A nervous predisposition to unrepaired DNA double strand breaks[J]. DNA Repair (Amst), 2013, 12(8): 588–599. DOI:10.1016/j.dnarep.2013.04.011 |
| [19] | POULTON C, OEGEMA R, HEIJSMAN D, et al. Progressive cerebellar atrophy and polyneuropathy:expanding the spectrum of PNKP mutations[J]. Neurogenetics, 2013, 14(1): 43–51. DOI:10.1007/s10048-012-0351-8 |
| [20] | BRAS J, ALONSO I, BARBOT C, et al. Mutations in PNKP cause recessive ataxia with oculomotor apraxia type 4[J]. Am J Hum Genet, 2015, 96(3): 474–479. DOI:10.1016/j.ajhg.2015.01.005 |
 2019, Vol. 45
2019, Vol. 45


