扩展功能
文章信息
- 冯敬媛, 李舒承, 王虹, 黄贤胜, 张爱文, 王文丰
- FENG Jingyuan, LI Shucheng, WANG Hong, HUANG Xiansheng, ZHANG Aiwen, WANG Wenfeng
- 姜黄素对慢性心力衰竭大鼠肾素-血管紧张素-醛固酮系统和心功能的改善作用
- Improvement effect of curcumin on Renin-angiotensin-aldosterone system and heart funotion in rats with chronic heart failure
- 吉林大学学报(医学版), 2019, 45(02): 325-330
- Journal of Jilin University (Medicine Edition), 2019, 45(02): 325-330
- 10.13481/j.1671-587x.20190219
-
文章历史
- 收稿日期: 2018-05-21
慢性心力衰竭是临床常见的一种心脏循环发生障碍导致心功能不全的心血管疾病[1-2]。研究[3-4]显示:慢性心力衰竭的患病率在1%以上,与患者年龄呈正相关关系,随着老龄化加剧,对慢性心力衰竭的研究显得更为重要。研究[5-7]显示:心力衰竭时机体往往会过度激活心脏肾素(Renin)-血管紧张素Ⅱ(AngⅡ)-醛固酮(ALD)系统(RAAS),调整患者的RAAS是治疗慢性心力衰竭的关键。因此,研究如何抑制RAAS的过度激活是治疗慢性心力衰竭的重要措施之一。
姜黄素是从姜科植物姜黄根茎中提取的一种多酚类物质,药理研究[8]显示:姜黄素具有抗炎、抗氧化、清除氧自由基、降血脂及抗动脉粥样硬化等多种药理学作用。国外研究[9]显示:姜黄素抑制组蛋白乙酰基转移酶P300(histone acetyltransferase p300, p300-HAT)活性的作用可以减轻实验动物模型心脏肥大和心力衰竭的发展,在治疗心力衰竭方面具有明显效果,但是目前尚无姜黄素作用于慢性心力衰竭的相关机制研究以及临床应用姜黄素治疗的报道。本研究探讨姜黄素对慢性心力衰竭大鼠RAAS的影响和心肌组织中血管紧张素受体1型(angiotensin type 1 receptor, AT1R)、血管紧张素转化酶(agniotensin-converting enzyme, ACE)mRNA和蛋白表达水平的影响,阐明其作用机制,为姜黄素的进一步实验研究以及临床推广提供理论依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器雄性SD大鼠50只,体质量200~240 g,购于上海斯莱克实验动物中心,动物合格证号:SCXK(沪)2012-0002。大鼠饲养于室温20℃~25℃,湿度40%~60%,通风良好,明暗光照交替12 h,标准饲料喂养,自由进食及饮水,适应环境2周。整个实验方案由承德医学院动物伦理委员会批准。
姜黄素(美国Sigma公司,纯度为99%),卡托普利片(汕头金石制药总厂),RT-PCR试剂盒(中国上海TaKaRa公司),Renin、AngⅡ和ALD的ELISA检测试剂盒(南京建成生物技术公司),ACE和AT1R抗体(美国Santa Cruz公司)。电泳仪(美国Bio-Rad公司),PCR仪(瑞士罗氏公司),高速离心机和超声心动图仪(德国西门子公司),动物心电图机(日本软隆公司)。
1.2 大鼠慢性心力衰竭模型的建立和实验分组随机选取40只雄性SD大鼠建立慢性心力衰竭大鼠模型[10]:术前禁食24 h,按50 mg·kg-1腹腔注射1%戊巴比妥钠麻醉大鼠,于手术台上在剑突下腹部正中将腹腔打开,在左冠状动脉前降支穿过1条5-0号丝线,结扎,观察心电图变化情况,即显示导联ST段表现为弓背向上抬高大于0.2 mV、且持续30 min以上即为成功建立慢性心力衰竭大鼠模型。另取10只SD大鼠作为假手术组,仅打开腹腔暴露左冠状动脉前降支但不做结扎。所有大鼠术后均连续3d进行腹腔注射青霉素20万单位,建模4周后,以左心室射血分数(left ventricular ejection fraction, LVEF)≤45%作为大鼠慢性心力衰竭模型成功。前期少数量预实验[11-12]结果显示:20~80 mg·kg-1姜黄素均对正常大鼠心功能造成一定影响,故将造模成功的大鼠随机分为模型组,低、中和高剂量姜黄素组,每组10只;低、中和高剂量姜黄素组大鼠分别灌胃给予20、40和80 mg·kg-1姜黄素,假手术组和模型组大鼠以等量生理盐水代替,连续给药30d。
1.3 心脏超声检测大鼠心功能分别于治疗前和给药30d后,利用超声心电图检测每组大鼠心功能情况,指标包括左心室舒张末内径(left ventricular end diastolic diameter, LVEDD)、左心室收缩末内径(left ventricular end systolic diameter, LVESD)和LVEF。
1.4 ELISA法检测大鼠血清中Renin、AngⅡ和ALD水平10%水合氯醛腹腔注射麻醉,腹主动脉取血,4℃低温下,以3000 r·min-1离心10 min,取血清,采用ELISA法检测各组大鼠血清中Renin、AngⅡ和ALD水平。实验步骤严格按试剂盒说明书进行,各组大鼠血清中Renin、AngⅡ和ALD水平的单位为ng·L-1。
1.5 RT-PCR法检测大鼠心肌组织中ACE和AT1R mRNA表达水平取大鼠心肌组织梗死区,Trizol法提取组织中总RNA,利用紫外分光光度计测定RNA水平,然后采用RT-PCR技术,按照试剂盒操作说明依次进行反转录反应,扩增,以GAPDH作为内参,采用Primer 3.0软件设计RT-PCR反应引物,测定ACE和AT1R mRNA的表达。引物序列:ACE上游引物5′-TTGACATGAGCAAGTTCCTG-3′,下游引物5′-CAAATCAGACTCGAGTGGCA-3′;AT1R上游引物5′-GCAATGGTCGGGACATAGTT-3′,下游引物5′-AGACCTGACTTGGCAGAGGA-3′;GAPDH上游引物5′-GTCGTAGCAAACCACCAAGC-3′,下游引物5′-TGTGGGTGAGGAGCACATAG-3′。以大鼠心肌组织中ACE和AT1R条带与GAPDH条带灰度值比值表示mRNA相对表达水平。
1.6 Western blotting法检测大鼠心肌组织中ACE和AT1R蛋白表达水平取各组大鼠心肌组织梗死区,RIPA裂解提取蛋白质,以BCA蛋白试剂盒定量蛋白浓度,蛋白上样量为50μg,12%SDS-PAGE凝胶电泳,移至硝酸纤维素膜,5%脱脂奶粉TBST封闭2h;分别加入ACE和AT1R抗体,4℃过夜,洗膜后加入二抗,孵育1h,采用Super ECL Plus超敏发光液显影,以ACE和AT1R条带与GAPDH条带灰度值比值表示目的蛋白相对表达水平。
1.7 统计学分析采用SPSS18.0统计软件进行统计学分析。各组大鼠LVEDD、LVESD和LVEF,血清中Renin、AngⅡ和ALD水平,心肌组织中AT1R和ACE mRNA及蛋白表达水平均以x±s表示;经方差齐性检验呈正态分布,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠心功能与假手术组比较,模型组,低、中和高剂量姜黄素组大鼠LVESD及LVEDD均明显升高(P < 0.05),而LVEF明显降低(P < 0.05);给药30d后,与模型组比较,低、中和高剂量姜黄素组大鼠LVESD和LVEDD明显降低,而LVEF明显升高(P < 0.05)。见表 1。
| (n=10, x±s) | |||
| Group | LVESD(d/mm) | LVEDD(d/mm) | LVEF(η/%) |
| Sham operation | 3.54±0.49 | 5.55±0.41 | 77.43±3.02 |
| Model | 6.11±0.81* | 7.38±0.67* | 40.91±3.44* |
| Curcumin | |||
| Low dose | 4.84±0.53*△ | 6.66±0.58*△ | 56.38±3.30*△ |
| Middle dose | 4.37±0.41*△ | 6.37±0.52*△ | 63.58±3.41*△ |
| High dose | 4.02±0.37*△ | 5.91±0.41*△ | 70.02±3.56*△ |
| *P < 0.05 compared with sham operation group;△P < 0.05 compared with model group. | |||
与假手术组比较,模型组,低、中和高剂量姜黄素组大鼠血清中Renin、AngⅡ及ALD水平均明显升高(P < 0.05);与模型组比较,低、中和高剂量姜黄素组大鼠血清中Renin、AngⅡ和ALD水平均明显降低(P < 0.05)。见表 2。
| [n=10, x±s, ρB/(ng·L-1)] | |||
| Group | Renin | AngⅡ | ALD |
| Sham operation | 4.31±0.89 | 419.24±27.28 | 107.08±8.86 |
| Model | 14.37±1.41* | 750.16±39.17* | 255.38±14.07* |
| Curcumin | |||
| Low dose | 11.20±1.03*△ | 603.46±42.11*△ | 200.43±12.36*△ |
| Middle dose | 8.07±0.98*△ | 552.11±30.25*△ | 166.15±10.53*△ |
| High dose | 6.32±0.75*△ | 504.28±28.91*△ | 129.47±9.18*△ |
| *P < 0.05 compared with sham opreation group;△P < 0.05 compared with model group. | |||
与假手术组比较,模型组,低、中和高剂量姜黄素组大鼠心肌组织中ACE及AT1R mRNA表达水平明显升高(P < 0.05);与模型组比较,低、中和高剂量姜黄素组大鼠心肌组织中ACE和AT1R mRNA表达水平明显降低(P < 0.05)。见图 1和表 3。
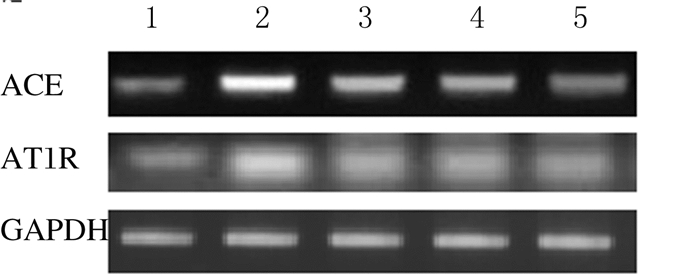
|
| Lane 1: Sham operation group; Lane 2: Model group; Lane 3-5: Low, middle, and high doses of curcumin groups. 图 1 各组大鼠心肌组织中ACE和AT1R mRNA表达电泳图 Fig. 1 Electrophoregram of expressions of ACE and AT1R mRNA in myocardium tissue of rats in various groups |
|
|
| (n=10, x±s) | ||
| Group | ACE mRNA | AT1R mRNA |
| Sham operation | 1.01±0.19 | 1.02±0.22 |
| Model | 2.46±0.45* | 2.61±0.47* |
| Curcumin | ||
| Low dose | 1.89±0.44*△ | 1.97±0.41*△ |
| Middle dose | 1.51±0.32*△ | 1.60±0.37*△ |
| High dose | 1.36±0.30*△ | 1.41±0.28*△ |
| *P < 0.05 compared with sham operation group;△P < 0.05 compared with model group. | ||
与假手术组比较,模型组,低、中和高剂量姜黄素组大鼠心肌组织中ACE及AT1R蛋白表达水平明显升高(P < 0.05);与模型组比较,低、中和高剂量姜黄素组大鼠心肌组织中ACE和AT1R蛋白表达水平明显降低(P < 0.05)。见图 2和表 4。
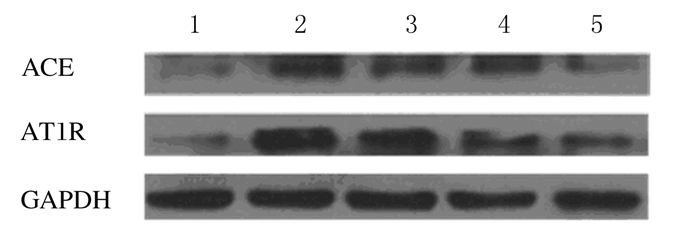
|
| Lane 1: Sham operation group; Lane 2: Model group; Lane 3-5: Low, middle, and high doses of curcumin groups. 图 2 各组大鼠心肌组织中ACE和AT1R蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of ACE and AT1R proteins in myocardium tissue of rats in various groups |
|
|
| (n=10, x±s) | ||
| Group | ACE | AT1R |
| Sham operation | 1.01±0.22 | 0.98±0.13 |
| Model | 2.71±0.43* | 1.99±0.37* |
| Curcumin | ||
| Low dose | 1.97±0.26*△ | 1.68±0.31*△ |
| Middle dose | 1.48±0.29*△ | 1.47±0.33*△ |
| High dose | 1.16±0.15*△ | 1.25±0.27*△ |
| *P < 0.05 compared with sham operation group;△P < 0.05 compared with model group. | ||
慢性心力衰竭是临床上一种复杂的综合征,引发因素很多,如高血压、冠心病、感染和心肌缺血等,而急性心肌缺血是促使心力衰竭加重的重要原因,已成为心血管疾病最主要的死亡原因[13-14]。目前临床治疗慢性心力衰竭的方法主要是一般药物治疗,主要有利尿剂、ACE/AngⅡ受体拮抗剂、活性胞嘧啶核苷脱氨酶和ALD拮抗剂,临床多采用他汀类药物治疗慢性心力衰竭,但近年来研究[15-18]显示调整患者RAAS是治疗慢性心力衰竭的关键。本研究结果显示:与假手术组比较,模型组,低、中和高剂量姜黄素组大鼠LVESD及LVEDD均明显升高,而LVEF明显降低,心电图变化对比证明成功建立慢性心力衰竭模型。
Renin是慢性心力衰竭的限速酶,是RSSA的引发物,可以降解血浆中的血管紧张素,将其分解为血管紧张素Ⅰ(AngⅠ),进而生成AngⅡ,而AngⅡ可作用于AT1R,引起血压升高、血管平滑肌收缩,使ALD分泌增加。ALD是调节电解质和细胞外液容量的激素,调节肾脏重吸收钠和排钾,维持体液容量和渗透压的平衡维持水平衡[19-20]。在心力衰竭患者心肌组织中可以检测到ACE和AT1R mRNA水平明显增加,提示心脏局部RSSA活性的增强和心力衰竭发展密切相关,ACE通过促进AngⅠ转化为AngⅡ与AT1R结合,发挥相应的生物学功能,临床研究[21-22]显示:体内RAAS与心脏交感神经系统的过度激活不仅能加重患者血流动力学紊乱,还对患者机体内的心肌细胞造成刺激和损害,从而加剧心肌重构,促使心力衰竭病情不断发展。本研究结果显示:慢性心力衰竭模型组大鼠血清中Renin、AngⅡ和ALD水平均明显升高,此反应与临床慢性心力衰竭病理情况下血液中Renin、AngⅡ和ALD水平变化相符,证明RAAS过度活动是慢性心力衰竭的特征之一。
AngⅡ或血小板衍生因子(platelet derived growth factor,PDGF)作用于心肌成纤维细胞的丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK),膜受体为AT1R,ALD有促AT1R mRNA的表达、使AT1R密度上调的作用,从而增强AngⅡ效应。成年动物和人的心脏、冠状动脉及主动脉存在血管紧张素原、Renin和ACE等构成成分,ACE途径可以生成AngⅡ,因此ACE水平可以反映AngⅡ水平变化。本研究结果显示:不同剂量姜黄素可明显改善慢性心力衰竭模型大鼠心功能,且有剂量依赖性。与模型组比较,低、中和高剂量姜黄素组大鼠血清中Renin、AngⅡ和ALD水平明显降低,心肌组织中ACE和AT1R mRNA及蛋白表达水平均明显降低,这可能是姜黄素发挥抑制慢性心力衰竭发生发展的机制之一,其通过作用于RAAS,整体调控RAAS的活性以治疗慢性心力衰竭,本研究为姜黄素治疗心血管疾病的应用提供了理论依据。
| [1] | 邱伯雍, 王永霞. 慢性心力衰竭流行病学及防治研究进展[J]. 中华实用诊断与治疗杂志, 2017, 31(6): 619–621. |
| [2] | 黄峻. 中国心力衰竭流行病学特点和防治策略[J]. 中华心脏与心律电子杂志, 2015, 3(2): 81–82. DOI:10.3877/cma.j.issn.2095-6568.2015.2.002 |
| [3] | 叶青, 程晓昱. 中医药治疗慢性心力衰竭研究概况[J]. 中医药临床杂志, 2016, 28(4): 464–467. |
| [4] | 周亚滨, 邬慧美, 孙静, 等. 慢性心力衰竭中医治疗进展[J]. 辽宁中医药大学学报, 2016, 18(1): 8–10. |
| [5] | SAYER G, BHAT G. The renin-angiotensin-aldosterone system and heart failure[J]. Cardiol Clin, 2014, 32(1): 21–32, ⅶ. |
| [6] | TAMARGO J, CABALLERO R, DELPÓN E. New therapeutic approaches for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors[J]. Cardiovasc Drugs Ther, 2018, 32(1): 99–119. DOI:10.1007/s10557-017-6767-5 |
| [7] | KHAN M S, FONAROW G C, KHAN H, et al. Renin-angiotensin blockade in heart failure with preserved ejection fraction:a systematic review and meta-analysis[J]. ESC Heart Fail, 2017, 4(4): 402–408. DOI:10.1002/ehf2.v4.4 |
| [8] | ZHANG C L, LI B Q, ZHANG X, et al. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients' PBMCs:potential role for STAT-3 and NF-kappaB signaling[J]. J Invest Dermatol, 2010, 130(8): 2110–2119. DOI:10.1038/jid.2010.86 |
| [9] | MARCU M G, JUNG Y J, LEE S, et al. Curcumin is an inhibitor of p300 histone acetylatransferase[J]. Med Chem, 2006, 2(2): 169–174. |
| [10] | 文建霞, 王建, 张璐, 等. 大鼠慢性心力衰竭模型研究现状[J]. 中国医院用药评价与分析, 2017, 17(9): 1157–1159. |
| [11] | 宋永周, 童九辉, 马维, 等. 姜黄素对软骨细胞氧化应激损伤的保护作用[J]. 河北医药, 2016, 38(23): 3525–3528. |
| [12] | 王晓静.MAPK信号转导通路在姜黄素预处理大鼠脑缺血再灌注损伤中的表达变化[D].蚌埠: 蚌埠医学院, 2010. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D667088 |
| [13] | ALLIDA S M, INGLIS S C, DAVIDSON P M, et al. A survey of views and opinions of health professionals managing thirst in chronic heart failure[J]. Contemp Nurse, 2016, 52(2/3): 244–252. |
| [14] | HÉNIN E, MEILLE C, BARBOLOSI D, et al. Revisiting dosing regimen using PK/PD modeling:the MODEL1 phase Ⅰ/Ⅱ trial of docetaxel plus epirubicin in metastatic breast cancer patients[J]. Breast Cancer Res Treat, 2016, 156(2): 331–341. DOI:10.1007/s10549-016-3760-9 |
| [15] | TOI M, SAEKI T, IWATA H, et al. A multicenter phase Ⅱ study of TSU-68, an oral multiple tyrosine kinase inhibitor, in combination with docetaxel in metastatic breast cancer patients with anthracycline resistance[J]. Breast Cancer, 2014, 21(1): 20–27. DOI:10.1007/s12282-012-0344-3 |
| [16] | UEDA S, KAWAKAMI H, NISHINA S, et al. Phase Ⅰ trial of 5-FU, docetaxel, and nedaplatin (UDON) combination therapy for recurrent or metastatic esophageal cancer[J]. Cancer Chemother Pharmacol, 2015, 76(2): 279–285. DOI:10.1007/s00280-015-2799-3 |
| [17] | LUKE J J, LORUSSO P, SHAPIRO G I, et al. ASP9853, an inhibitor of inducible nitric oxide synthase dimerization, in combination with docetaxel:preclinical investigation and a Phase Ⅰ study in advanced solid tumors[J]. Cancer Chemother Pharmacol, 2016, 77(3): 549–558. DOI:10.1007/s00280-016-2967-0 |
| [18] | LORUSSO P M, INFANTE J R, KIM K B, et al. A phase Ⅰ dose-escalation study of selumetinib in combination with docetaxel or dacarbazine in patients with advanced solid tumors[J]. BMC Cancer, 2017, 17(1): 173. DOI:10.1186/s12885-017-3143-6 |
| [19] | PITT B, BAKRIS G L, BUSHINSKY D A, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors[J]. Eur J Heart Fail, 2015, 17(10): 1057–1065. DOI:10.1002/ejhf.402 |
| [20] | WEIR M R, BAKRIS G L, BUSHINSKY D A, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors[J]. N Engl J Med, 2015, 372(3): 211–221. DOI:10.1056/NEJMoa1410853 |
| [21] | WEIR M R, BAKRIS G L, GROSS C, et al. Treatment with patiromer decreases aldosterone in patients with chronic kidney disease and hyperkalemia on renin-angiotensin system inhibitors[J]. Kidney Int, 2016, 90(3): 696–704. DOI:10.1016/j.kint.2016.04.019 |
| [22] | PERGOLA P E, SPIEGEL D M, WARREN S, et al. Patiromer lowers serum potassium when taken without food:comparison to dosing with food from an open-label, randomized, parallel group hyperkalemia study[J]. Am J Nephrol, 2017, 46(4): 323–332. DOI:10.1159/000481270 |
 2019, Vol. 45
2019, Vol. 45


