扩展功能
文章信息
- 王利红, 张影, 兰坤, 张海玉, 曹肖琲, 李善玉
- WANG Lihong, ZHANG Ying, LAN Kun, ZHANG Haiyu, CAO Xiaobei, LI Shanyu
- 黄芪多糖对哮喘模型小鼠肺组织炎症的抑制作用及其机制
- Inhibitory effect of astragalus polysaccharides on pulmonary inflammation in asthma model mice and its mechanism
- 吉林大学学报(医学版), 2019, 45(02): 313-318
- Journal of Jilin University (Medicine Edition), 2019, 45(02): 313-318
- 10.13481/j.1671-587x.20190217
-
文章历史
- 收稿日期: 2018-11-15
2. 天津市儿童医院儿科, 天津 300074
2. Department of Pediatrics, Children's Hospital of Tianjin City, Tianjin 300074, China
黄芪多糖(astragalus polysaccharides,APS)是传统中药黄芪的主要活性成分,具有抗氧化、抗炎、抗衰老和调节免疫等药理作用[1-2]。范文彤[3]通过研究证实APS可改善小鼠T细胞功能,并能提高化疗药物所致免疫抑制小鼠的吞噬细胞功能。近年来研究[4]显示:儿童哮喘发病率逐年增加,寻找儿童适用新型抗哮喘药物具有重要意义。目前认为Th1/Th2和Th17/Treg细胞比例失衡及相关细胞因子异常表达是哮喘的主要发病机制[5],而APS可改善机体的T细胞免疫功能,在自身免疫性疾病和炎症应答中作用明确,理论上应可发挥良好的抗哮喘作用[6]。近年来国内针对APS治疗哮喘方面的研究[7-8]显示:APS可通过调整相关免疫细胞表型,从细胞免疫途径缓解哮喘症状,但目前针对不同相对分子质量APS治疗哮喘的研究较少。本实验对比观察不同相对分子质量的APS对哮喘小鼠的疗效,检测T细胞不同亚群和细胞因子水平,探讨APS对哮喘的治疗作用机制。
1 材料与方法 1.1 实验动物、主要试剂和仪器30只无特殊病原体(specific pathogen free,SPF)B级BALB/c雌性小鼠购自吉林大学基础医学院动物实验中心,饲养于SPF级实验室,周龄6~8周,体质量18~22g,动物合格证号:SCXK(吉)2013-0001。卵白蛋白(ovalbumin, OVA)、生理盐水和低、中及高相对分子质量APS粉末(吉林大学公共卫生学院, 相对分子质量分别为4500、15000和30000)。瑞士-吉姆萨染色液,CD4+T细胞分离磁珠试剂盒,包含白细胞介素4(interleukin-4, IL-4)、γ-干扰素(γ-interferon, IFN-γ)、白细胞介素17(interleukin-17, IL-17)和白细胞介素10(interleukin-10, IL-10)等特异性捕获抗体的Cytometric Bead Array试剂盒(美国BD公司)。雾化器(德国百瑞公司),4℃离心机(芬兰Thermo公司),CO2培养箱(日本SANYO公司),流式细胞仪(美国BD公司)。
1.2 实验动物分组和处理方法30只BALB/c小鼠随机分为正常对照组,模型组,低、中和高相对分子质量APS组,每组6只。①模型组:于第0、7和14天小鼠腹腔注射0.1mL致敏液(100 μg OVA+2 mg Al(OH)3),于第21天开始将小鼠放置雾化箱内,2% OVA混悬液雾化激发,每日1次,每次45min,雾化7d制备哮喘小鼠模型;②低、中和高相对分子质量APS组:小鼠哮喘模型制备同模型组,于每天激发前30 min对各组小鼠分别腹腔注射0.1 mL低、中和高相对分子质量APS;③正常对照组:采用等量生理盐水代替雾化致敏液及腹腔注射。于末次激发后48 h处死各组小鼠。
1.3 标本采集和处理① 血清标本留取:眼球取血,分离血清,-20℃冻存;②小鼠肺组织病理切片HE染色:采用颈椎脱臼法处死小鼠,处死后开胸,结扎左主支气管,切取左肺叶,10%中性甲醛固定, 经脱水、石蜡包埋、切片,HE染色,光学显微镜下观察;③小鼠支气管肺泡灌洗液(bronchoalvelor lavage fluid, BALF)的收集:固定小鼠颈前气管,静脉留置针穿刺,注射生理盐水,回收,重复3次,离心,取上清,-20℃冻存;④流式细胞小球微阵列术(Cytometric Bead Array, CBA)法检测:采用CBA法检测各组小鼠血清和BALF中IL-4及IFN-γ水平,显微镜下计数BALF中白细胞(while blood cells, WBC)、中性粒细胞(neutrophil, Neu)及嗜酸性粒细胞(eosnophils, Eos)数量;⑤细胞实验和细胞培养:取各组小鼠脾脏,研磨,PBS冲洗,磁珠分选提纯CD4+ T细胞,37℃、5% CO2环境下细胞培养;⑥流式细胞术和CBA法检测:流式细胞术检测各组小鼠Th1、Th2、Th17和Treg细胞比例,CBA试剂盒检测36h时细胞培养上清液中IL-4、IFN-γ、IL-17和IL-10水平。具体操作同参考文献[9]。
1.4 统计学分析采用SPSS17.0统计软件进行统计学分析。各组小鼠血清和BALF中IL-4及IFN-γ水平、BALF中WBC总数和炎症细胞分类计数,Th1、Th2、Th17和Treg细胞比例及培养上清液中IL-4、IFN-γ、IL-17和IL-10水平以x±s表示。各组数据间比较采用单样本Kolmogorov-Smirnov法分别进行正态检验,多组间比较采用单因素方差分析。以P<0.05为差异有统计学意义。
2 结果 2.1 小鼠行为学改变模型组小鼠在雾化激发过程中逐渐出现打喷嚏、抓耳挠腮和呼吸急促等症状,然后出现乏力、少动、体质量减轻和毛色光泽减退等;低、中和高相对分子质量APS组小鼠上述症状较模型组有不同程度的改善,正常对照组小鼠未出现上述表现。
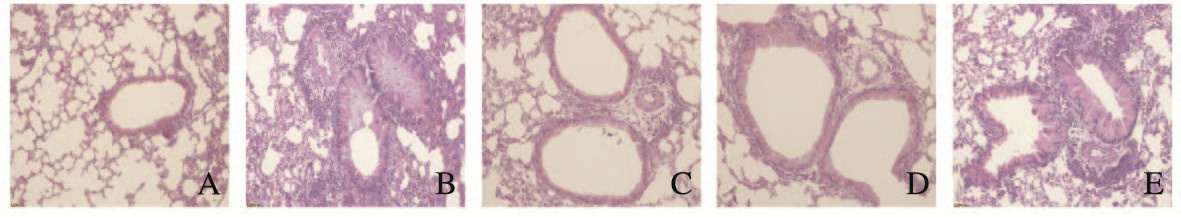
2.2 各组小鼠肺组织形态和炎症表现模型组小鼠细支气管、肺间质和肺泡腔内可见大量炎性细胞浸润,气道上皮不完整,黏膜水肿,平滑肌增厚,管腔狭窄,气道内可见大量黏液和黏液栓。与模型组比较,APS组小鼠肺组织结构相对较规整,气道平滑肌轻微增厚,炎症细胞浸润少;APS组哮喘小鼠肺组织结构和炎性浸润均有改善,以低相对分子质量APS组最明显。正常对照组小鼠肺组织未见明显异常。见图 1(插页四)。
|
| A: Normal control group; B: Model group; C:APS-low group; D: APS-middle group; E: APS-high group. 图 1 各组小鼠肺组织形态表现(HE,×400) Fig. 1 Morphology of lung tissue of mice in various groups (HE, ×400) |
|
|
与正常对照组比较,模型组小鼠血清和BALF中IL-4水平明显升高(P < 0.05), IFN-γ水平明显降低(P < 0.05);与模型组比较,APS组小鼠血清和BALF中IL-4水平明显降低(P < 0.05), IFN-γ水平明显升高(P < 0.05);与低相对分子质量APS组比较,中和高相对分子质量APS组小鼠血清和BALF中IL-4水平明显升高(P < 0.05),小鼠血清和BALF中IFN-γ水平明显降低(P < 0.05),但中和高相对分子质量APS组间比较差异无统计学意义(P>0.05)。见表 1。
| [n=6,x±s, ρB/(ng·L-1)] | |||||
| Group | Serum | BALF | |||
| IL-4 | IFN -γ | IL-4 | IFN -γ | ||
| Normal control | 3.94±0.12 | 89.48±1.39 | 4.80±0.62 | 94.53±1.32 | |
| Model | 13.63±0.61* | 22.45±0.81* | 22.24±0.59* | 19.60±0.68* | |
| APS-low | 6.03±0.76△ | 33.38±0.76△ | 8.87±0.45△ | 38.03±0.13△ | |
| APS-middle | 7.94±0.37△# | 27.48±1.38△# | 12.18±0.74△# | 28.53±1.52△# | |
| APS-high | 9.05±0.81△# | 21.22±0.74# | 13.84±0.79△# | 24.02±1.10△# | |
| *P < 0.05 compared with normal control group; △P < 0.05 compared with model group; #P < 0.05 compared with APS-low group. | |||||
与正常对照组比较,模型组小鼠BALF中WBC总数和Eos及Neu比例明显升高(P < 0.05);与模型组比较,APS组小鼠BALF中WBC总数和Eos及Neu比例均明显降低(P < 0.05);与低相对分子质量APS组比较,中和高相对分子质量APS组小鼠BALF中WBC总数和Eos及Neu比例明显升高(P < 0.05);小鼠BALF中WBC总数在中和高相对分子质量APS组间比较差异无统计学意义(P>0.05)。见表 2。
| (n=6, x±s) | |||
| Group | WBC(×106 L-1) | Eos(η/%) | Neu(η/%) |
| Normal control | 1.02±0.92 | 2.07±0.13 | 0.89±0.11 |
| Model | 38.50±1.39* | 32.46±1.73* | 5.14±0.37* |
| APS-low | 8.59±0.62△ | 9.74±0.40△ | 1.86±0.27△ |
| APS-middle | 11.61±1.52△# | 12.84±0.40△# | 3.88±0.28△# |
| APS-high | 14.64±0.78△# | 16.96±0.54△# | 3.92±0.29△# |
| *P < 0.05 compared with normal control group; △P < 0.05 compared with model group; #P < 0.05 compared with APS-low group. | |||
与正常对照组比较,模型组小鼠Th1和Treg细胞比例明显降低(P < 0.05),Th2和Th17细胞比例明显升高(P < 0.05);与模型组比较,APS组小鼠Th1和Treg细胞比例明显升高(P < 0.05),Th2和Th17细胞比例明显降低(P < 0.05);3种不同相对分子质量APS组之间比较差异有统计学意义(P < 0.05),其中低相对分子质量APS组作用更明显。见表 3。
| (n=3, x±s, η/%) | ||||
| Group | Th2 | Th1 | Th17 | Treg |
| Normal control | 6.03±0.18 | 24.10±1.56 | 1.01±0.42 | 13.77±0.78 |
| Model | 12.45±0.64* | 10.20±0.57* | 4.30±0.47* | 7.34±0.68* |
| APS-low | 3.27±0.37△ | 15.42±0.40△ | 0.99±0.21△ | 11.98±0.18△ |
| APS-middle | 5.10±0.42△# | 14.60±0.57△# | 1.29±0.13△# | 9.78±0.17△# |
| APS-high | 7.18±0.37△#○ | 12.95±0.21△#○ | 1.44±0.22△#○ | 9.33±0.24△#○ |
| *P < 0.05 compared with normal control group; △ P < 0.05 compared with model group; #P < 0.05 compared with APS-low group; ○ P < 0.05 compared with APS-middle group. |
||||
与正常对照组比较,模型组小鼠细胞上清液中IL-4和IL-17水平明显升高(P < 0.05),IFN-γ和IL-10水平明显降低(P < 0.05);与模型组比较,APS组小鼠细胞上清液中IL-4和IL-17水平明显降低(P < 0.05),IFN-γ和IL-10水平明显升高(P < 0.05);不同相对分子质量APS组之间比较差异无统计学意义(P>0.05)。见表 4。
| [n=6,x±s, ρB/(ng·L-1)] | ||||
| Group | IL-4 | IFN-γ | IL-17 | IL-10 |
| Normal control | 279.51±6.37 | 1 442.58±12.71 | 1.53±0.15 | 2 352.05±10.92 |
| Model | 1 011.08±23.79* | 752.65±7.60* | 14.45±0.26* | 551.62±8.13* |
| APS-low | 501.14±13.06△ | 800.04±8.08△ | 7.90±0.28△ | 988.58±10.22△ |
| APS-middle | 791.36±20.11△ | 953.09±9.50△ | 10.25±0.28△ | 1 582.44±12.07△ |
| APS-high | 635.11±16.60△ | 933.51±9.32△ | 9.88±0.30△ | 1 253.85±14.02△ |
| *P < 0.05 compared with normal control group; △P < 0.05 compared with model group. | ||||
APS是黄芪中最重要的活性成分,现代医学研究[1-3]发现:APS可促进免疫细胞分化,调节细胞因子表达,有效改善机体免疫功能;临床研究[10]表明:APS可增强B细胞、T细胞和NK细胞等多种免疫细胞的活性,改善机体特异性和非特异性免疫状态。颜爱等[11]发现:给予环磷酰胺免疫抑制模型小鼠APS治疗后,血清中IL-2、IL-10和IFN-γ水平明显提高,免疫抑制状态改善。APS具有免疫调节和免疫增强双重作用,从哮喘发生的免疫学机制的角度,可推测其对于哮喘患者的应用前景广阔。
哮喘发病机制复杂,与免疫、遗传和环境等多种因素相关[12],目前多认为CD4+T细胞对外界刺激的特异性免疫应答是该病触发点[13],Th1/Th2及Th17/Treg细胞比例失衡是哮喘发病的重要基础[14],而呼吸道Neu和Eos堆积是引发哮喘的关键[15]。IFN-γ和IL-4分别是Th1和Th2细胞分化的特征性细胞因子。IFN-γ是一种抗炎介质,可抑制IgE合成、促进IgG合成,减弱特异性IgE介导的Ⅰ型超敏反应,还能抑制Eos炎性浸润作用[16]。IL-4是一种促炎因子,一方面可促进B细胞增殖分化为浆细胞,产生IgE,促进IgE介导体液免疫应答[17-19];另一方面,通过B细胞对T细胞抗原提呈作用,促进炎症部位Eos的聚集和浸润,参与气道炎症形成[16]。Th17和Treg细胞是CD4+ T细胞的2个新亚型,分别起着促进和抑制炎症反应的作用,在自身免疫性疾病、感染性疾病和肿瘤的病理过程中发挥重要作用。IL-17为Th17细胞分泌的一种细胞因子,被认为是Neu浸润的一个触发者,并可调节Eos和巨噬细胞性炎症[20-22], 因此,测定IL-17水平可以反映组织的炎症浸润程度。IL-10是Treg细胞在免疫应答中起主要作用的免疫抑制因子, 可有效减轻机体炎症反应。正常状态下,来源于共同初始T细胞的Th1、Th2、Th17和Treg细胞亚群,功能上相互抑制共同维系了机体免疫稳态,当机体受到异常抗原刺激时,出现了CD4+ T细胞分化向Th2和Th17细胞偏移,导致哮喘发生。
本研究结果显示:APS可降低哮喘小鼠体内促炎细胞(Th2细胞、Th17细胞、WBC、Eos和Neu)及细胞因子(IL-4和IL-17)水平,并提高Th1和Treg细胞比例及IFN-γ和IL-10水平,从而纠正以Th2和Th17细胞为主的过度免疫炎症反应,减少肺组织中Neu和Eos炎性浸润,对肺组织损伤具有保护作用。低相对分子质量APS较中和高相对分子质量APS作用更明显,原因可能是其相对分子质量小,分子表面多糖结构暴露相对完全,能更有效地与特定细胞膜受体结合而发挥作用,或从基因水平上调控相关免疫分子的表达[23],但具体分子机制和最适剂量有待进一步研究。
综上所述,APS可有效调节Th1/Th2、Th17/Treg细胞及相关细胞因子的产生,对哮喘小鼠肺组织的炎症具有抑制作用,其中低相对分子质量APS有可能研发成为机制明确、效果良好的治疗哮喘和调节免疫的药物。
| [1] | 黄家林, 张勇. 黄芪多糖抗炎免疫作用机制研究进展[J]. 中西医结合心脑血管病杂志, 2013, 11(11): 1374–1376. DOI:10.3969/j.issn.1672-1349.2013.11.054 |
| [2] | DU X, ZHAO B, LI J, et al. Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice[J]. Int Immunopharmacol, 2012, 14(4): 463–470. |
| [3] | 范文彤. 黄芪多糖对小鼠免疫功能的药理学实验研究[J]. 中国当代医药, 2018, 25(3): 10–14. DOI:10.3969/j.issn.1674-4721.2018.03.004 |
| [4] | ROSINE N. Natural childbirth:do maternal microbes prevent asthma?[J]. Kinderkrankenschwester Organ Der Sektion Kinderkrankenpflege, 2015, 34(5): 197. |
| [5] | RAY A, COHN L. Altering the Th1/Th2 balance as a therapeutic strategy in asthmatic diseases[J]. Curr Opinion Investigat Drugs, 2000, 1(4): 442. |
| [6] | 邢琼琼, 赵霞. 黄芪多糖治疗哮喘作用机制研究述评[J]. 河南中医, 2018, 38(10): 1610–1613. |
| [7] | 刘璟, 游进. 黄芪多糖在变应性哮喘大鼠模型中对树突状细胞的免疫干预作用[J]. 中国生物制品学杂志, 2018, 31(2): 140–144. |
| [8] | 邱镞文, 高伟, 吴炬, 等. 黄芪多糖注射液对哮喘患者支气管灌洗液或痰液炎症细胞计数和相关因子水平的影响[J]. 解放军预防医学杂志, 2018, 36(6): 746–749. |
| [9] | 曹肖琲.黄芪多糖对哮喘小鼠CD4+T细胞作用的研究[D].长春: 吉林大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10183-1014292342.htm |
| [10] | LIU Q, YAO Y, YU Y, et al. Correction:Astragalus polysaccharides attenuate postburn sepsis via inhibiting negative immunoregulation of CD4+CD25 high T Cells[J]. PLoS ONE, 2011, 6(6): e19811. DOI:10.1371/journal.pone.0019811 |
| [11] | 颜爱, 李波, 李润成, 等. 香菇多糖和黄芪多糖对免疫抑制小鼠免疫功能调节的研究[J]. 中国免疫学杂志, 2012, 28(11): 999–1005. DOI:10.3969/j.issn.1000-484X.2012.11.008 |
| [12] | 朱晓洁, 王珺, 张予阳. 多机制多通路参与哮喘新说[J]. 沈阳药科大学学报, 2014, 31(4): 325–330. |
| [13] | AGNES H, HAÏFA M, ANISSA B, et al. Transcriptional characteristics of CD4+T cells in young asthmatic children:RORC and FOXP3 axis[J]. J Inflammation Res, 2011, 4(1): 139–146. |
| [14] | DE VOOGHT V, SMULDERS S, HAENEN S, et al. Neutrophil and eosinophil granulocytes as key players in a mouse model of chemical-induced asthma[J]. Toxicol Sci, 2013, 131(2): 406–418. DOI:10.1093/toxsci/kfs308 |
| [15] | VARGA E M, WACHHOLZ P, NOURI-ARIA K T, et al. T cells from human allergen-induced late asthmatic responses express IL-12 receptor beta2 subunit mRNA and respond to IL-12 in vitro[J]. J Immunol, 2000, 165(5): 2877–2885. DOI:10.4049/jimmunol.165.5.2877 |
| [16] | 姚丽敏, 高丽. 支气管哮喘发病机制研究进展[J]. 新疆中医药, 2017, 35(1): 43–46. |
| [17] | WALSH G M. Anti-IL-4/-13 based therapy in asthma[J]. Expert Opin Emerg Drugs, 2015, 20(3): 349–352. DOI:10.1517/14728214.2015.1050377 |
| [18] | KHAN D A. Allergic rhinitis and asthma:Epidemiology and common pathophysiology[J]. Allergy Asthma Proc, 2014, 35(5): 357–361. DOI:10.2500/aap.2014.35.3794 |
| [19] | 孟泳, 崔应麟, 李彬. 厚朴麻黄汤对哮喘小鼠血清IgE、IL-4、IL-13及半胱氨酰白三烯水平的影响[J]. 郑州大学学报:医学版, 2017, 52(2): 193–196. |
| [20] | GUPTA R K, GUPTA K, DWIVEDI P D. Pathophysiology of IL-33 and IL-17 in allergic disorders[J]. Cytokine Growth Factor Rev, 2017, 38: 22–36. DOI:10.1016/j.cytogfr.2017.09.005 |
| [21] | 赵霞, 盛慧萍, 杨岩, 等. 慢性乙型肝炎患者外周血IL-17细胞因子水平及其临床意义[J]. 西安交通大学学报:医学版, 2017, 38(1): 83–87. |
| [22] | 杨浦娟, 黄祎, 刘华宝. IL-17与肝脏疾病的相关性[J]. 临床肝胆病杂志, 2017, 33(9): 1810–1814. DOI:10.3969/j.issn.1001-5256.2017.09.041 |
| [23] | 王金磊, 李承德, 孙宏伟, 等. 黄芪多糖抑制NF-κB/MAPK信号通路和改善哮喘大鼠气道炎症的作用[J]. 中国药理学报, 2016, 32(4): 489–493. |
 2019, Vol. 45
2019, Vol. 45


