扩展功能
文章信息
- 张静, 赵岩, 蔡恩博, 祝洪艳, 李平亚, 刘金平
- ZHANG Jing, ZHAO Yan, CAI Enbo, ZHU Hongyan, LI Pingya, LIU Jinping
- 人参倍半萜有效部位对S180荷瘤小鼠的抗肿瘤活性及其作用机制
- Anti-tumor activity of sesquiterpenoids from ginseng in S180 tumor-bearing mice and its mechanism
- 吉林大学学报(医学版), 2019, 45(02): 280-285
- Journal of Jilin University (Medicine Edition), 2019, 45(02): 280-285
- 10.13481/j.1671-587x.20190212
-
文章历史
- 收稿日期: 2018-06-29
2. 吉林大学药学院新药研究室, 吉林 长春, 130021
2. New Drug Research Laboratory, School of Pharmacy, Jilin University, Changchun 130021, China
人参为五加科植物人参的根及根茎,其味甘、性温、微苦,具有补脾益肺、生津止渴、安神益智之功效,是中国最常用的、最重要的和最珍贵的传统中药材之一[1]。人参在临床上具有广泛的药用价值,常用于心血管、肿瘤和神经系统等疾病的治疗[2]。目前,人参已成为肿瘤辅助治疗的热点药物之一[3]。有关人参抗肿瘤作用的物质基础和作用机制研究主要集中在人参皂苷方面,如人参皂苷Rg3、Rh2及二醇组皂苷元原人参二醇(glycol saponin protopanaxadiol, PPD)等稀有皂苷及苷元[4-7]。针对人参中所含的另一种重要活性组分人参倍半萜有效部位(sesguiterpenoids from ginseng, SPG),本项目组开展了一系列研究[8-11],明确了这种由21种倍半萜组成的有效部位的抗抑郁活性和抗急性肝损伤作用,并证实其作用机制与抗氧化和抗炎作用有关联。研究[12]显示炎症反应在癌症的发生和发展过程中起着重要的作用。本研究以S180荷瘤小鼠为模型,对SPG的抗肿瘤作用及潜在机制进行研究,旨在为SPG的进一步开发提供依据。
1 材料与方法 1.1 人参倍半萜人参(4年,中国抚松)经ASI Spe-ed SFE-2超临界CO2萃取仪提取,得到人参脂溶性部位,采用分馏法分离得到SPG;采用气相色谱-质谱(GC-MS)分析仪分析SPG中倍半萜类成分和含量,共检出21种倍半萜化合物,其中含量达66%[8]。
1.2 主要试剂和仪器5-氟尿嘧啶(5-FU)购于九鼎化工科技有限公司,白细胞介素2(IL-2)、肿瘤坏死因子α(TNF-α)和血管内皮生长因子(VEGF)ELISA试剂盒购于美国R & D公司,丙氨酸氨基转移酶(ALT)、天冬氨酸氨基转移酶(AST)和血尿素氮(BUN)购于北京安迪华泰科技有限公司,苏木精-伊红(HE)染料试剂盒购于南京建成生物工程研究所,抗细胞凋亡因子(Bcl-2)、p38对丝裂原活化蛋白激酶(p38MAPK)和p-p38对丝裂原活化蛋白激酶(p-p38MAPK)购于英国ABCAM有限公司,VEGF购自北京宝森生物技术有限公司。电子分析天平购自常熟百灵天平仪器有限公司,酶标仪购于美国Bio-Tek公司,ASI Spe-ed SFE-2超临界CO2萃取系统购于美国ASI公司,GC-MS分析仪购于安捷伦科技有限公司。
1.3 实验动物50只ICR级雄性小鼠,体质量为18~22g,购于长春亿斯实验动物技术有限公司,动物许可证号:SCXK(吉)2016-0003,饲养环境温度为(23±2)℃、湿度为50%~60%,给予小鼠12 h光照和12 h黑暗,用普通饲料和水喂养。在实验进行前,所有小鼠适应性饲养1周[13]。
1.4 实验动物模型建立和处理小鼠S180细胞购于吉林省肿瘤医院研究所,每只小鼠于腹腔中接种0.2 mL S180细胞,饲养5~7d,在无菌条件下从小鼠腹腔中取出乳白色腹水,用无菌生理盐水按照一定的比例稀释成细胞密度为1×105 mL-1,连续传2代,取出腹水瘤细胞悬液0.2 mL接种于小鼠右上肢腋部皮下[14-15]。24 h后随机将接种小鼠分为模型组、5-FU组(20 mg·kg-1,ip)、低剂量SPG组(大豆油溶解,2.5 mg·kg-1,ig)和高剂量SPG组(大豆油溶解,10 mg·kg-1,ig),每组8只; 另选8只ICR级雄性小鼠作为空白对照组,空白对照组和模型组小鼠给予同体积大豆油灌胃,连续给药14 d,期间观察各组小鼠肿瘤形成、生长发育情况和精神状态。
1.5 观察荷瘤小鼠生长情况并计算抑瘤率实验期间,每次给药前及解剖前称小鼠体质量,评估小鼠体质量差异。小鼠末次给药后,禁食不禁水24 h,单侧眼球取血,颈椎脱臼处死,将小鼠解剖,剥离肿瘤组织并称质量,计算抑瘤率[13]。抑瘤率=(模型组小鼠平均瘤质量-给药组小鼠平均瘤质量)/模型组小鼠平均瘤质量×100%。
1.6 检测荷瘤小鼠血清指标取小鼠全血,离心取上清液,于-80℃冰箱中保存。按照试剂盒说明书操作步骤检测各组小鼠血清中IL-2、TNF-α、VEGF、AST、ALT和BUN水平[14]。
1.7 HE染色观察荷瘤小鼠肿瘤组织形态表现将肿瘤组织固定于10%的甲醛溶液中24 h,并进行常规脱色、包埋,切成4μm薄片,行HE染色。于400倍光学显微镜下观察肿瘤组织病理形态表现。以细胞排序情况、细胞完整度、细胞核数量和坏死区域大小评价肿瘤组织生长及凋亡情况[15-16]。
1.8 Western blotting法测定小鼠肿瘤组织中Bcl-2、VEGF、p38MAPK和p-p38MAPK蛋白表达水平采用Western blotting法测定Bcl-2和VEGF在肿瘤组织中的表达水平,并通过检测p38MAPK和p-p38MAPK的蛋白表达水平,确定p38MAPK蛋白通道,以各蛋白条带与内参β-actin条带的灰度值比值表示蛋白的表达水平[16]。
1.9 统计学分析采用SPSS 17.0统计软件进行统计学分析。各组小鼠体质量和血清学指标以x±s表示,小鼠肿瘤组织中相关蛋白表达水平采用Prism5(GraphPad)软件作图,各组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠体质量、生长情况和抑瘤率与空白对照组比较,5-FU组小鼠体质量增加幅度降低(P < 0.05),SPG组小鼠体质量差异无统计学意义(P>0.05);与模型组比较,5-FU组小鼠体质量增加幅度明显降低(P < 0.05);与5-FU组比较,SPG组小鼠体质量增加幅度明显升高(P < 0.05)。SPG组小鼠精神状态及生长情况明显优于5-FU组,并且与空白对照组比较无明显差别。与模型组比较,5-FU和SPG小鼠肿瘤质量明显降低(P < 0.05),且高剂量SPG组抑瘤率达到76.29%。见表 1。
| (n=8, x±s) | ||||||
| Group | Dose (mg·kg-1) |
Weight(m/g) | Increase (m/g) |
Tumor | ||
| Pre-treatment | Post-treatment | Weight(m/g) | Inhibitory rate(η/%) | |||
| Blank control | 0 | 26.08±0.69 | 31.77±0.99 | 5.59±0.51 | 0 | 0 |
| Model | 0 | 26.28±0.78 | 31.96±1.06 | 5.68±0.49 | 1.35±0.67 | 0 |
| 5-FU | 20.0 | 25.92±1.81 | 31.04±1.79 | 5.10±0.56*△ | 0.29±0.11△ | 78.51 |
| Low dose of SPG | 2.5 | 25.58±2.49 | 31.16±1.33 | 5.53±0.58# | 0.51±0.23△ | 61.89 |
| High dose of SPG | 10.0 | 26.08±1.82 | 31.17±2.01 | 5.58±0.60# | 0.32±0.14△ | 76.29 |
| *P < 0.05 vs blank control group; △P < 0.05 vs model group; #P < 0.05, ##P < 0.01 vs 5-FU group. | ||||||
与空白对照组比较,模型组小鼠血清中BUN水平明显升高(P < 0.01),5-FU组小鼠血清中AST和ALT水平明显升高(P < 0.01);与模型组比较,SPG组小鼠血清中ALT和BUN水平明显降低(P < 0.01),高剂量SPG组小鼠血清中AST水平明显降低(P < 0.05)。与5-FU组比较,SPG组小鼠血清中AST、ALT和BUN水平明显降低(P < 0.01)。见表 2。
| (n=8, x±s) | ||||
| Group | Dose(mg·kg-1) | ALT[ρB/(ng·L-1)] | AST[ρB/(ng·L-1)] | BUN[cB/(pmol·L-1)] |
| Blank control | 0 | 49.81±4.05 | 182.18±12.31 | 596.92±86.15 |
| Model | 0 | 58.48±6.09 | 198.56±17.07 | 652.53±91.27** |
| 5-FU | 20.0 | 70.91±4.08*△△ | 248.17±14.01*△△ | 797.45±48.48*△△ |
| Low dose of SPG | 2.5 | 52.24±6.55△△# | 184.67±11.06# | 617.89±85.17△△# |
| High dose of SPG | 10.0 | 55.37±4.78△△# | 188.17±16.66*△# | 623.45±68.11△△# |
| *P < 0.01 vs blank control group; △P < 0.05, △△P < 0.01 vs model group; # P < 0.01 vs 5-FU group. | ||||
与空白对照组比较,模型组小鼠血清中IL-2和TNF-α水平明显降低(P < 0.01),低和高剂量SPG组小鼠血清中TNF-α水平明显升高(P < 0.01),模型组小鼠血清中VEGF水平明显升高(P < 0.01)。与模型组比较,5-FU组和各剂量SPG组小鼠血清中IL-2和TNF-α水平明显升高(P < 0.05或P < 0.01),而VEGF水平明显降低(P < 0.01);与5-FU组比较,高剂量SPG组小鼠血清中IL-2和TNF-α水平明显升高(P < 0.01),而VEGF水平明显降低(P < 0.01),低剂量SPG组小鼠血清中TNF-α水平明显升高(P < 0.05)。见表 3。
| [n=8, x±s, ρB/(ng·L-1)] | ||||
| Group | dose(mg·kg-1) | IL-2 | TNF-α | VEGF |
| Blank control | 0 | 159.19±10.38 | 917.48±119.29 | 282.18±18.31 |
| Model | 0 | 118.82±18.31** | 798.68±125.97** | 401.00±33.47** |
| 5-FU | 20.0 | 150.86±11.51△△ | 929.41±79.56△ | 304.11±46.69*△△ |
| Low dose of SPG | 2.5 | 158.68±6.69△△ | 1 041.10±112.65**△△# | 263.51±18.16△△ |
| High dose of SPG | 10.0 | 171.26±16.26**△△## | 1 087.11±132.56**△△## | 238.33±34.86△△## |
| *P < 0.05 ** P < 0.01 vs blank control group; △P < 0.05, △△P < 0.01 vs model group; #P < 0.05, ##P < 0.01 vs 5-FU group. | ||||
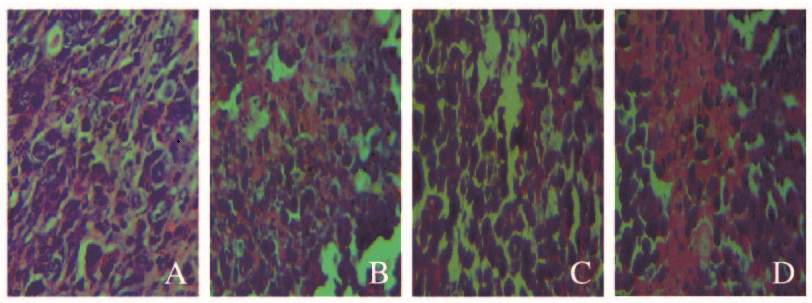
模型组肿瘤细胞排列整齐紧密,可见大量细胞核,且细胞生长状态良好;5-FU组肿瘤细胞核数量明显减少,肿瘤组织疏松,并且可见大面积组织坏死区域;低剂量SPG组肿瘤细胞核数量明显减少,细胞排列不规则;高剂量SPG组肿瘤细胞凋亡显著,出现大面积组织坏死区域。见图 1(插页三)。
|
| A:Model group; B:5-FU group; C:Low dose of SPG group; D:High dose of SPG group. 图 1 各组小鼠肿瘤组织病理学表现(HE,×400) Fig. 1 Pathology of tumor tissue of mice in various groups(HE, ×400) |
|
|
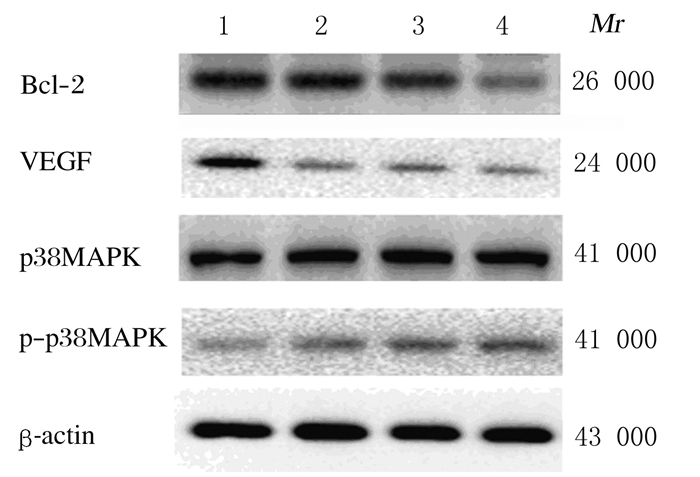
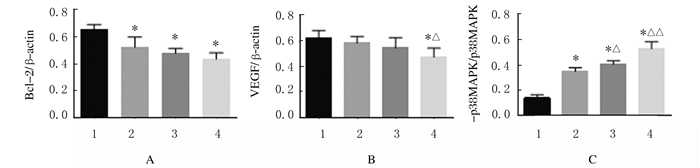
与模型组比较,5-FU组和SPG组小鼠肿瘤组织中Bcl-2蛋白表达水平明显降低(P < 0.01),高剂量SPG组VEGF表达水平明显降低(P < 0.01),5-FU组和SPG组小鼠肿瘤组织中p-p38MAPK/p38MAPK比值明显升高(P < 0.01);与5-FU组比较,高剂量SPG组小鼠肿瘤组织中VEGF表达水平明显降低(P < 0.05), 各剂量SPG组小鼠肿瘤组织中p38MAPK/p-p38MAPK比值明显升高(P < 0.05或P < 0.01)。见图 2和3。
|
| Lane1: Model group; Lane2: 5-FU group; Lane3: Low dose of SPG group; Lane4: High dose of SPG group. 图 2 各组小鼠肿瘤组织中相关蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of related proteins in tumor tissue of mice in various groups |
|
|
|
| A: Bcl-2;B: VEGF; C: p-p38MAPK/p38MAPK.1:Model group; 2:5-FU group; 3:Low dose of SPG group; 4:High dose of SPG group.*P<0.01 vs model group; △P<0.05, △△P < 0.01 vs 5-FU group. 图 3 各组小鼠肿瘤组织中相关蛋白的表达水平 Fig. 3 Expression levels of related proteins in tumor tissue of mice in various groups |
|
|
人参具有抗肿瘤作用,LEE等[17]研究发现:人参脂溶性成分对肿瘤细胞表现出明显的抑制作用。本研究探讨了SPG对S180荷瘤小鼠的抗肿瘤作用,结果显示:SPG具有明显的抗肿瘤作用,高剂量(10.0 mg·kg-1)SPG组抑瘤率可达76.29%,接近于5-FU组小鼠抑瘤率。血清中AST和ALT水平代表药物或疾病对肝脏的损伤程度,BUN代表药物对肾脏损伤程度,此3项指标越高,表明肝脏和肾脏损伤程度越深[5]。本实验结果表明:SPG能够降低肿瘤对荷瘤小鼠肝脏和肾脏的损伤程度。此外,研究[15]显示:免疫细胞的细胞因子在机体抵抗肿瘤过程中起到关键作用。TNF-α是一种抗肿瘤细胞因子,具有许多生物活性,如对肿瘤细胞的直接毒性、抗肿瘤免疫反应的激活和肿瘤血管的选择性损伤[18]。VEGF是肿瘤细胞生长和繁殖的重要因素之一,其能够为血管的生成提供营养物质,可刺激肿瘤血管的生成[19]。IL-2在肿瘤细胞生长的特异性免疫反应中起重要作用,并且促进先天性和适应性免疫应答[15]。本实验结果显示:与5-FU组和模型组比较,SPG能够明显提高荷瘤小鼠血清中IL-2和TNF-α水平,降低血清中VEGF水平。在肿瘤的发生发展过程中,Bcl-2在调控细胞凋亡过程中起到重要作用,其过度表达会造成细胞逃脱凋亡[20]。VEGF是肿瘤血管生成的一个重要因子,其在肿瘤细胞增殖与迁移过程中发挥重要作用[13]。本实验结果显示:SPG能够明显降低荷瘤小鼠肿瘤组织中Bcl-2和VEGF蛋白表达水平。p38MAPK蛋白通道是丝裂原活化蛋白激酶(MAPK)家族的经典途径之一,其在细胞增殖、分化和凋亡方面发挥重要作用[21-22]。p-p38MAPK通过刺激p53磷酸化以促进原癌基因C-Myc的表达,进一步促进TNF-α的表达增加,最终促进肿瘤细胞的凋亡[23]。本实验结果表明:SPG增加了荷瘤小鼠肿瘤组织中p-p38MAPK蛋白表达水平。
综上所述,SPG具有明显的抗肿瘤活性,其抗肿瘤作用可能是通过降低Bcl-2和VEGF表达以及激活p38MAPK蛋白通道实现的。SPG有希望成为具有预防肿瘤作用的食品添加剂或治疗肿瘤的药物。
| [1] | 刘鲲, 刘娜, 刘苾川, 等. 人参抗肿瘤药理研究[J]. 光明中医, 2016, 31(1): 140–143. DOI:10.3969/j.issn.1003-8914.2016.01.074 |
| [2] | 冯彦. 人参药理作用及临床应用研究进展[J]. 中医临床研究, 2013, 5(6): 121–122. |
| [3] | 罗林明, 石雅宁, 姜懿纳, 等. 人参抗肿瘤作用的有效成分及其机制研究进展[J]. 中草药, 2017, 48(3): 582–596. |
| [4] | ZOU M J, WANG J, GAO J D, et al. Phosphoproteomic analysis of the antitumor effects of ginsenoside Rg3 in human breast cancer cells:[J]. Oncol Lett, 2018, 15(3): 2889–2898. |
| [5] | GAO Q R, ZHENG J H. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A[J]. Cell Prolif, 2018, 51(3): e12438. DOI:10.1111/cpr.2018.51.issue-3 |
| [6] | ZHANG H, XU H L, WANG Y C, et al. 20(S)-protopanaxadiol-induced apoptosis in MCF-7 breast cancer cell line through the inhibition of PI3K/AKT/mTOR signaling pathway[J]. Int J Mol Sci, 2018, 19(4): E1053. DOI:10.3390/ijms19041053 |
| [7] | CHEN G L, LI H J, GAO Y G, et al. Flavored black ginseng exhibited antitumor activity via improving immune function and inducing apoptosis[J]. Food Funct, 2017, 8(5): 1880–1889. DOI:10.1039/C6FO01870J |
| [8] | GE W, LI H, ZHAO Y, et al. Study on antidepressant activity of sesquiterpenoids from ginseng root[J]. J Funct Foods, 2017, 33: 261–267. DOI:10.1016/j.jff.2017.03.057 |
| [9] | WANG W D, LIU X F, LIU J P, et al. Sesquiterpenoids from the root of Panax Ginseng attenuates lipopolysaccharide-induced depressive-like behavior through the BDNF/TrkB and Sirt 1/NF-κB signaling pathways[J]. Agric Food Chem, 2018, 66(1): 265–271. DOI:10.1021/acs.jafc.7b04835 |
| [10] | WANG W D, ZHANG Y G, LI H J, et al. Protective effects of sesquiterpenoids from the root of panax ginseng on fulminant liver injury induced by lipopolysaccharide/d-galactosamine[J]. J Agric Food Chem, 2018, 66(29): 7758–7763. DOI:10.1021/acs.jafc.8b02627 |
| [11] | WANG W D, WANG S J, LIU J P, et al. Sesquiterpenoids from the root of panax ginseng protect CCl4-induced acute liver injury by anti-inflammatory and anti-oxidative capabilities in mice[J]. Biomed Pharmacother, 2018, 102: 412–419. DOI:10.1016/j.biopha.2018.02.041 |
| [12] | 姚静, 胡容, 郭青龙. 炎症与癌症的发生发展[J]. 药物生物技术, 2011, 18(4): 372–376. |
| [13] | LIN M Z, LI H J, ZHAO Y, et al. 2-Naphthoic acid ergosterol ester, an ergosterol derivative, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis[J]. Steroids, 2017, 122: 9–15. DOI:10.1016/j.steroids.2017.03.007 |
| [14] | 林明珠, 赵岩, 蔡恩博, 等. β-谷甾醇对H(22)荷瘤小鼠体内抗肿瘤作用[J]. 中国公共卫生, 2017, 33(12): 1797–1800. DOI:10.11847/zgggws2017-33-12-31 |
| [15] | LI W, XU Q, HE Y F, et al. Anti-tumor effect of steamed codonopsis lanceolata in H22 tumor-bearing mice and its possible mechanism[J]. Nutrients, 2015, 7(10): 8294–8307. DOI:10.3390/nu7105395 |
| [16] | MO L F, CHEN Y F, LI W J, et al. Anti-tumor effects of (1→3)-β-d-glucan from Saccharomyces cerevisiae in S180 tumor-bearing mice[J]. Int Biol Macromol, 2017, 95: 385–387. DOI:10.1016/j.ijbiomac.2016.10.106 |
| [17] | LEE S D, PARK S K, LEE E S, et al. A lipid-soluble red ginseng extract inhibits the growth of human lung tumor xenografts in nude mice[J]. J Med Food, 2010, 13(1): 1–5. |
| [18] | YOSHIOKA Y, TSUTSUMI Y, IKEMIZU S, et al. Optimal site-specific PEGylation of mutant TNF-alpha improves its antitumor potency[J]. BiochemBiophysRes Commun, 2004, 315(4): 808–814. DOI:10.1016/j.bbrc.2004.01.125 |
| [19] | FUJIWARA N, FRIEDMAN S L, GOOSSENS N, et al. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine[J]. J Hepatol, 2018, 68(3): 526–549. DOI:10.1016/j.jhep.2017.09.016 |
| [20] | YANG B, XIAO B, SUN T Y. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice[J]. Int J Biol Macromol, 2013, 62: 287–290. DOI:10.1016/j.ijbiomac.2013.09.016 |
| [21] | WU D D, GAO Y F, CHEN L X, et al. Anti-tumor effects of a novel chimeric peptide on S180 and H22 xenografts bearing nude mice[J]. Peptides, 2010, 31(5): 850–864. DOI:10.1016/j.peptides.2010.01.007 |
| [22] | ZHU Q W, CHEN J H, LI Q, et al. Antitumor activity of polysaccharide from Laminaria japonica on mice bearing H22 liver cancer[J]. Int J Biol Macromol, 2016, 92: 156–158. DOI:10.1016/j.ijbiomac.2016.06.090 |
| [23] | 张文娟, 蒋伟哲, 蓝献丽, 等. 五谷虫提取物对H22肝癌细胞荷瘤小鼠的抗肿瘤机制研究[J]. 广西医学, 2017, 39(2): 215–219. |
 2019, Vol. 45
2019, Vol. 45


