扩展功能
文章信息
- 毕雪婷, 申玉芹, 徐晓薇, 李文洁, 王卓然, 林崇韬
- BI Xueting, SHEN Yuqin, XU Xiaowei, LI Wenjie, WANG Zhuoran, LIN Chongtao
- 寡核苷酸YW002对人牙周膜干细胞增殖、周期、凋亡和早期成骨分化的影响
- Effects of oligodeoxynucleotide YW002 on proliferation, cell cycle, apoptosis and early osteogenic differentiation of human periodontal ligament stem cells
- 吉林大学学报(医学版), 2019, 45(02): 273-279
- Journal of Jilin University (Medicine Edition), 2019, 45(02): 273-279
- 10.13481/j.1671-587x.20190211
-
文章历史
- 收稿日期: 2018-06-15
2. 吉林省牙发育及颌骨重塑与再生重点实验室, 吉林 长春 130021
2. Key Laboratory of Tooth Development and Bone Remodeling and Regeneration, Jilin Province, Changchun 130021, China
牙周炎是由牙菌斑生物膜等因素引起的牙周组织慢性感染性疾病,可导致牙槽骨吸收,进而引起牙齿脱落,影响咀嚼功能甚至影响生活质量。目前,牙周基础治疗难以完全有效地恢复已丧失的牙槽骨组织,针对这一临床问题,促进牙周组织再生受到关注,通过植入生长因子、支架材料及种子细胞实现牙槽骨重塑促进牙周组织修复,已成为牙周临床研究的关键。
寡核苷酸(oligodeoxynucleotide, ODN)是指含有50个以下核苷酸单体的多核苷酸链,是一些以未甲基化的核苷酸基序为核心的DNA序列[1]。有研究[2-3]显示:ODN可通过Toll样受体9(Toll-like receptor 9, TLR9)调控破骨细胞的形成和分化,通过被内吞小体膜上的TLR9受体识别并结合,通过MyD88-IRAK-TRAF6途径激活核因子κB(nuclear factor-κB, NF-κB)和MAP激酶,从而启动抗感染免疫应答,维持机体自身的稳定[4]。近年也有研究[5]通过ODN与复合纳米材料来提高免疫刺激水平。同时ODN与骨改建也有密切联系,ODN可通过TLR9调控破骨细胞的形成和分化[6],通过上调白细胞介素12(interleukin-12, IL-12)的表达抑制NF-κB受体活化因子配体的产生从而抑制破骨细胞分化[7]。本课题组近年来一直致力于ODN与牙槽骨重塑相关分析的研究工作,在前期实验[8-9]中发现某些特定序列ODN,如免疫抑制型ODN MT01具有较为显著的促进成骨作用,可抑制实验性大鼠牙周炎导致的牙槽骨吸收。本文作者选取一种命名为YW002的免疫刺激型ODN,与免疫抑制型ODN MT01进行对比,探讨不同类型ODN对细胞生物活性影响的异同。目前国内外文献尚无有关ODN YW002对人牙周膜干细胞(human periodontal ligament stem cells, hPDLSCs)生物活性影响的报道。本文作者选取hPDLSCs作为种子细胞,以免疫抑制型ODN MT01作为对照,从细胞增殖、细胞周期、凋亡和成骨分化等方面,探讨ODN YW002对hPDLSCs的影响,旨在阐明ODN YW002对hPDLSCs的调控作用,为将ODN YW002应用于后续的牙周组织再生研究奠定实验基础。
1 材料与方法 1.1 主要试剂和仪器高糖DMEM培养基、胰蛋白酶、青霉素/链霉素双抗和D-Hank’ s液(Hyclone公司,美国),胎牛血清(Gibco公司,美国),细胞周期检测试剂盒和Annexin-Ⅴ-FITC细胞凋亡试剂盒(上海七海复泰生物科技有限公司,中国),二喹啉甲酸(bicinchoninic acid, BCA)和碱性磷酸酶(alkaline phosphates, ALP)试剂盒(南京建成生物工程研究所,中国),ODN YW002和ODN MT01(吉林大学基础医学院分子生物学教研室设计,日本TaKaRa公司合成)。CO2恒温细胞培养箱(Sanyo公司,日本),倒置显微镜(Olympus公司,日本),高端多功能酶标仪(Tecan公司,奥地利)。
1.2 hPDLSCs原代培养和形态观察利用组织块原代培养法进行hPDLSCs原代培养。收集吉林大学口腔医院外科门诊因正畸治疗需要拔出的健康前磨牙,患者年龄10~20岁,拔出后立刻放入含有双抗的D-Hank’ s液中,轻晃3~5min,冰浴下立刻送至超净台内,镊子夹住牙颈部并根面向上,用5mL无菌注射器抽取生理盐水反复冲洗牙根面,用锐利的龈下刮治器刮取根中1/3的牙周膜,将组织剪成约1mm×1mm×1mm的小块,每块间距约5mm,均匀接种在培养瓶的瓶底,倒置培养瓶,缓慢注入3mL培养液(20%血清,1%双抗)且避免组织块脱落,孵育3~4h后组织贴附,将培养瓶慢慢放平静置培养,之后每3d更换1次培养液,加4~5mL的培养液。待细胞长满80%~90%时按1:2传代,选择第3~5代细胞进行以下实验,应用倒置显微镜观察细胞形态。
1.3 合成ODN YW002和ODN MT01ODN YW002和ODN MT01由吉林大学基础医学院分子生物学实验室设计,日本TaKaRa公司合成并全硫代修饰,每条序列用无菌PBS稀释至工作浓度(1mg·L-1),-20℃保存备用。YW002序列:5′-TCGCGAACGTTCGCCGCGTTCGAACGCGG-3′,MT01序列:5′-ACCCCCTCTACCCCCTCTACCCCCTCT-3′。
1.4 MTT法检测hPDLSCs细胞增殖活性取第3~5代生长状态良好的对数生长期细胞,以每孔5000个细胞接种于96孔板。实验分为空白对照组、ODN MT01组和ODN YW002组,待细胞孵育24 h完全贴壁后,每组分别加入PBS和ODN,再孵育1、3和5 d,孵育结束后,按照每孔20 μL加MTT液(5 g·L-1),避光孵育4h,弃液,每孔加入150 μL DMSO充分溶解,于酶标仪490 nm波长处检测吸光度(A)值,以A值表示细胞增殖活性。
1.5 流式细胞术检测细胞凋亡率取第3~5代生长状态良好的对数生长期细胞,以每孔1.2×105个细胞的密度接种于6孔板。实验分为PBS空白对照组、ODN MT01组和ODN YW002组。培养24和48h后消化收集细胞,PBS冲洗2次,每孔加入500μL的Binding Buffer悬浮细胞,再每孔加入5μL Annexin Ⅴ-EGFP室温、避光、反应15 min,每孔加入5 μL碘化丙啶避光、冰浴5 min,在1 h内应用流式细胞仪检测细胞凋亡率。
1.6 流式细胞术检测细胞周期取第3~5代生长状态良好的对数生长期细胞,以每孔1.2×105个细胞的密度接种于6孔板。实验分为PBS空白对照组、ODN MT01组和ODN YW002组。培养24 h后收集细胞,预冷PBS洗涤2次,70%乙醇4℃进行固定2 h,每孔加入500 μL碘化丙啶进行染色,37℃避光孵育30 min,在1 h内应用流式细胞仪检测各细胞周期中hPDLSCs百分比。
1.7 磷酸苯二钠微板法检测细胞ALP活性取第3~5代生长状态良好的对数生长期细胞,以每孔1.2×105个细胞接种于6孔板。实验分为PBS空白对照组、ODN MT01组和ODN YW002组。培养1、3和5d后,按照产品说明书操作,检测各组ALP活性和蛋白浓度,分别于酶标仪波长520和562nm处检测各孔A值,并计算ALP活性和总蛋白浓度。ALP活性(U·g-1)=(测定A值-空白A值)/(标准A值-空白A值)×酚标准品浓度/待测样本浓度,总蛋白浓度(mg·L-1)=(测定A值-空白A值)/(标准A值-空白A值)×标准品浓度×样品稀释倍数。
1.8 统计学分析采用SPSS 20.0统计软件进行统计学分析。各组hPDLSCs的细胞增殖活性、细胞凋亡率和细胞周期百分比均以x±s表示,观察指标符合正态分布,多组间比较采用单因素方差分析,两两比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
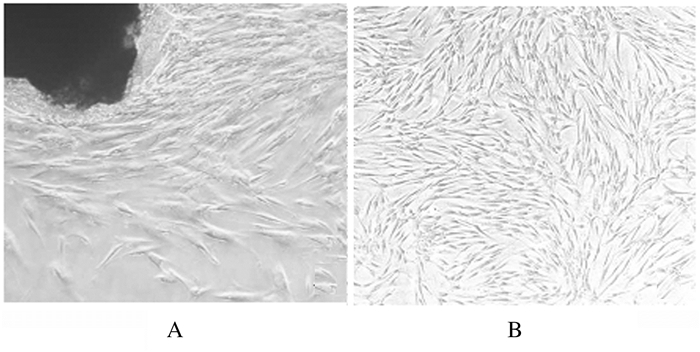
2 结果 2.1 培养hPDLSCs的形态表现倒置显微镜下:hPDLSCs生长状态良好,呈长梭形,胞浆透明,胞体丰满,胞突细长,核为卵圆形,位于胞体中央,似成纤维样细胞。见图 1。
|
| A:×100;B:×40. 图 1 原代(A)和第三代(B)人牙周膜干细胞的形态 Fig. 1 Morphology of primary (A) and third generation (B) human periodontal ligament stem cells |
|
|
与空白对照组比较,培养1和3d时OND MT01组和ODN YW002组hPDLSCs增殖活性升高(P < 0.01);培养5d时ODN YW002组hPDLSCs增殖活性升高,但差异无统计学意义(P>0.05)。与ODN MT01组比较,培养1d时ODN YW002组hPDLSCs活性下降(P < 0.05);培养3和5d时hPDLSCs增殖活性升高,但差异无统计学意义(P>0.05)。见表 1。
| (n=4, x±s) | ||||
| Group | A value | |||
| (t/d) | 1 | 3 | 5 | |
| Blank control | 0.178±0.002 | 0.328±0.012 | 0.424±0.002 | |
| ODN MT01 | 0.211±0.020* | 0.367±0.023** | 0.458±0.020** | |
| ODN YW002 | 0.201±0.014**△ | 0.367±0.023** | 0.478±0.047 | |
| * P < 0.05, * * P < 0.01 compared with blank control group;△ P < 0.05 compared with ODN MT01 group. | ||||
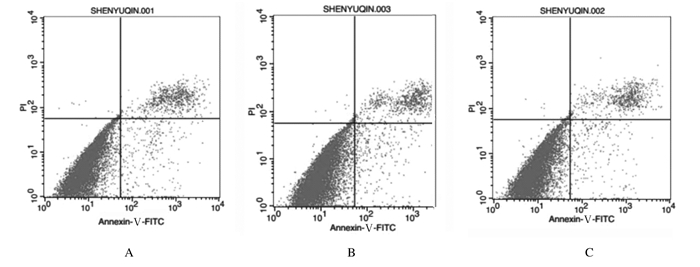
ODN MT01和ODN YW002作用后1 d,与空白对照组比较,ODN MT01组和ODN YW002组hPDLSCs早期和晚期凋亡率均降低(P < 0.05);且ODN YW002组早期凋亡率低于ODN MT01组(P < 0.05)。作用后2 d,与空白对照比较,ODN MT01组hPDLSCs早期凋亡率升高(P < 0.05),晚期凋亡率下调(P < 0.05);ODN YW002组hPDLSCs早期和晚期凋亡率均降低,但差异无统计学意义(P>0.05);且ODN YW002组hPDLSCs早期和晚期凋亡率均低于ODN MT01组(P < 0.05或P < 0.01)。见图 2、3和表 2。

|
| A: Blank control group; B: ODN MT01 group; C: ODN YW002 group. 图 2 培养1 d时各组hPDLSCs早期和晚期凋亡率 Fig. 2 Early and late apoptotic rates of hPDLSCs in various groups at 1 d after culture |
|
|
|
| A: Blank control group; B: ODN MT01 group; C: ODN YW002 group. 图 3 培养2 d时各组hPDLSCs早期和晚期凋亡率 Fig. 3 Early and late apoptotic rates of hPDLSCs in various groups at 2 d after culture |
|
|
| (n=3, x±s, η/%) | ||||||
| Group | Early apoptotic rate | Late apoptotic rate | ||||
| (t/d) | 1 | 2 | 1 | 2 | ||
| Blank control | 4.03±0.41 | 2.89±0.14 | 10.22±1.20 | 7.37±0.53 | ||
| ODN MT01 | 2.35±0.72* | 3.13±0.27* | 4.84±0.55** | 7.33±0.51* | ||
| ODN YW002 | 1.78±0.51**△ | 2.48±0.44△ | 5.00±1.58** | 6.63±0.41△△ | ||
| * P < 0.05, * * P < 0.01, compared with blank control group; △ P < 0.05, △△ P < 0.01 compared with ODN MT01 group. | ||||||
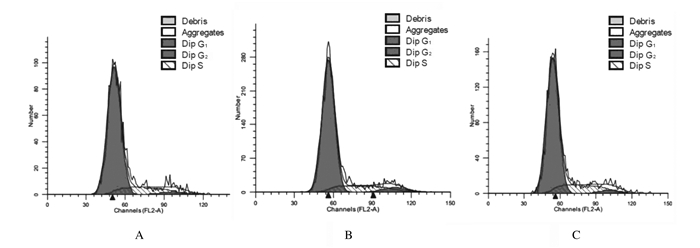
ODN MT01和ODN YW002作用后1 d,与空白对照组比较,ODN MT01组和ODN YW002组G0/G1期hPDLSCs百分比降低,S期hPDLSCs百分比升高,但差异均无统计学意义(P>0.05)。与ODN MT01组比较,ODN YW002组G0/G1期hPDLSCs百分比降低,S期hPDLSCs百分比升高,但差异均无统计学意义(P>0.05)。见表 3和图 4。
| (n=3, x±s, η/%) | |||
| Group | Percentage of hPDLSCs | ||
| G0/G1 | S | G2/M | |
| Blank control | 79.87±8.03 | 15.98±1.61 | 4.15±0.42 |
| ODN MT01 | 78.44±6.79 | 15.90±1.38 | 5.67±0.49 |
| ODN YW002 | 76.96±7.23 | 18.89±1.78 | 4.15±0.39 |
|
| A: Blank control group; B: ODN MT01 group; C: ODN YW002 group. 图 4 培养1 d时各组hPDLSCs的细胞周期 Fig. 4 Cell cycles of hPDLSCs in various groups at 1 d after culture |
|
|
与空白对照组比较,ODN MT01组培养1和3d时hPDLSCs中ALP活性明显升高(P < 0.05或P < 0.01),培养5d时hPDLSCs中ALP活性降低(P>0.05);与空白对照组比较,ODN YW002组培养1、3和5d时hPDLSCs中ALP活性明显升高(P < 0.01);与ODN MT01组比较,ODN YW002组培养1和5d时hPDLSCs中ALP活性升高(P < 0.05或P < 0.01),培养3d时hPDLSCs中ALP活性降低(P < 0.05)。见表 4。
| [n=3, x±s, λB/(U·g-1)] | ||||
| Group | ALP | |||
| (t/d) | 1 | 3 | 5 | |
| Blank control | 157.171±4.911 | 105.948±2.442 | 123.097±2.463 | |
| ODN MT01 | 172.669±0.885* | 121.976±0.801** | 120.979±1.679 | |
| ODN YW002 | 179.064±3.934**△ | 118.970±1.707**△ | 134.838±1.919**△△ | |
| * P < 0.05, * * P < 0.01 compared with blank control group; △ P < 0.05, △△ P < 0.01 compared with ODN MT01 group. | ||||
牙周膜干细胞被认为是理想的调控牙周组织再生的种子细胞。2004年SEO等[10]首次在人牙周膜组织中分离出hPDLSCs,并发现其具有干细胞的特性,能在一定条件下向成骨细胞和成纤维细胞等分化,在牙周组织再生中具有重要的作用。牙周膜干细胞的提取方法有组织块法、酶联合消化法和酶解组织块法。酶联合消化法中酶消化时间通常难确定,酶解组织块法原代培养成功率低,所以本实验选择原代耗时较长的组织块法,优点是能获得较高的提纯率,并经过多次传代贴壁培养纯化细胞。原代培养观察可见:培养7~20d后,组织块周围爬出细胞,并呈放射状向四周生长;3~4周达到瓶底80%或细胞局部过于密集影响生长时传代培养。本研究结果显示:传代初的前5代细胞活性较高,细胞增殖能力强。
对牙周膜干细胞生物活性、凋亡和细胞周期的影响是促进牙周组织再生的关键因素。有研究[11]显示:在炎症状态下成骨样细胞阻滞于G1期,抑制细胞增殖,影响自体组织修复。本研究结果显示:ODN YW002促进hPDLSCs增殖,在趋势上显示其缩短了G1期,使细胞周期向S期进展,这可能是其促进细胞活跃增殖的原因。研究[12]显示:ODN MT01可以促进牙龈卟啉单胞菌感染下的成骨样细胞进入S期。本研究结果与上述结论相一致。
本研究结果显示:ODN YW002能有效抑制hPDLSCs的凋亡。细胞凋亡与牙周炎有着密切关联,牙周炎主要细菌毒性产物脂多糖可以通过诱导肿瘤坏死因子释放而活化凋亡相关因子Caspase-3和Caspase-8,进而促进细胞凋亡[13]。有研究[14]将含ODN的重组质粒导入小鼠颗粒细胞,Caspase-3和Caspase-8等促凋亡基因mRNA水平明显降低,与本实验结果显示ODN抑制凋亡的现象一致。但ODN对不同细胞的作用有差异,有研究[15]将ODN导入癌症细胞后通过上调凋亡相关因子Caspases促进肿瘤细胞的凋亡,而GHAZI等[16]研究显示ODN对细胞系的存活无明显影响。
诱导牙周膜干细胞向成骨细胞分化使其具备成骨功能是促进牙周组织再生的关键,本实验初步证实:ODN YW002具有调控hPDLSCs成骨分化的潜能。本实验选取细胞成骨分化的早期指标ALP进行检测。在骨矿化过程中,无机焦磷酸对羟磷灰石具有抑制作用,ALP通过水解无机焦磷酸,从而利于骨矿化,ALP也是骨形成的特异性标志[17]。本实验结果显示:ODN YW002作用后细胞中ALP活性较空白对照组升高,这与本文作者前期实验[18-19]结果相一致,也与国外学者[20]研究报道的结果相一致,即ODN可以通过靶向TGF-β1调控通路上的骨保护素(osteoprotegenin, OPG),使OPG表达上调,促进间充质细胞成骨向分化。
综上所述,ODN YW002通过促进hPDLSCs的增殖,抑制hPDLSCs早期和晚期凋亡,进而对hPDLSCs的生物活性产生影响;在调控细胞生物活性的同时,ODN YW002可诱导hPDLSCs中早期成骨标志性因子ALP活性升高,提示ODN YW002可能具有促进hPDLSCs成骨的作用。这一假设有待后续在基因水平及功能方向进一步验证。
| [1] | TOKUNAGA T, YAMAMOTO H, SHIMADA S, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity[J]. J Natl Cancer Inst, 1984, 72(4): 955–962. |
| [2] | LIN T, PAJARINEN J, NABESHIMA A, et al. Orthopaedic wear particle-induced bone loss and exogenous macrophage infiltration is mitigated by local infusion of NF-κB decoy oligodeoxynucleotide[J]. J Biomed Mater Res A, 2017, 105(11): 3169–3175. DOI:10.1002/jbm.a.36169 |
| [3] | YU X Q, WANG Y H, LIN J, et al. Lipopolysaccharides-induced suppression of innate-like B cell apoptosis is enhanced by CpG oligodeoxynucleotide and requires toll-like receptors 2 and 4[J]. PLoS ONE, 2016, 11(11): e0165862. DOI:10.1371/journal.pone.0165862 |
| [4] | HUANG Q J, YANG J H, LIN Y, et al. Differential regulation of interleukin 1 receptor and toll-like receptor signaling by MEKK3[J]. Nat Immunol, 2004, 5(1): 98–103. DOI:10.1038/ni1014 |
| [5] | HANAGATA N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies[J]. Int J Nanomed, 2017, 16(12): 515–531. |
| [6] | AMCHESLAVSKY A, HEMMI H, AKIRA S, et al. Differential contribution of osteoclast-and osteoblast-lineage cells to CpG-oligodeoxynucleotide (CpG-ODN) modulation of osteoclastogenesis[J]. J Bone Miner Res, 2005, 20(9): 1692–1699. DOI:10.1359/JBMR.050515 |
| [7] | AMCHESLAVSKY A, ZOU W, BAR-SHAVIT Z. Toll-like receptor 9 regulates tumor necrosis factor-alpha expression by different mechanisms implications for osteoclastogenesis[J]. J Biol Chem, 2004, 279(52): 54039–54045. DOI:10.1074/jbc.M409138200 |
| [8] | SHEN Y, FENG Z, LIN C, et al. An oligodeoxynucleotide that induces differentiation of bone marrow mesenchymal stem cells to osteoblasts in vitro and reduces alveolar bone loss in rats with periodontitis[J]. Int J Mol Sci, 2012, 13(3): 2877–2892. DOI:10.3390/ijms13032877 |
| [9] | 高涵, 申玉芹, 刘引, 等. MT01对感染状态人成骨样细胞内ALP活性及mRNA表达的影响[J]. 口腔医学研究, 2016, 32(6): 580–583. |
| [10] | SEO B M, MIURA M, GRONTHOS S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament[J]. Lancet, 2004, 364(9429): 149–155. DOI:10.1016/S0140-6736(04)16627-0 |
| [11] | 潘春玲, 赵海礁, 谭丽思, 等. 牙龈卟啉单胞菌感染MG63细胞对细胞周期的影响[J]. 口腔医学研究, 2015, 31(9): 863–866. |
| [12] | 于海蛟, 申玉芹, 刘引, 等. 特定序列寡核苷酸MT01对牙龈卟啉单胞菌感染的成骨细胞的增殖、细胞周期及凋亡的影响[J]. 华西口腔医学杂志, 2015, 33(6): 617–621. |
| [13] | ZHOU Y, ZHANG H, ZHANG G, et al. Calcitonin gene related peptide reduces Porphyromonas gingivalis LPS induced TNF α release and apoptosis in osteoblasts[J]. Mol Med Rep, 2018, 17(2): 3246–3254. |
| [14] | 阳美霞, 张虹亮, 李蓝祁, 等. 含CpG-ODN基序重组抑制素质粒的构建及其对小鼠颗粒细胞相关凋亡基因表达的影响[J]. 畜牧兽医学报, 2018, 49(8): 1625–1632. |
| [15] | HE X Y, LIU B Y, WU J L, et al. A dual macrophage targeting nanovector for delivery of oligodeoxynucleotides to overcome cancer-associated immunosuppression[J]. ACS Appl Mater Interfaces, 2017, 9(49): 42566–42576. DOI:10.1021/acsami.7b13594 |
| [16] | GHAZI B, THONNART N, BAGOT M, et al. KIR3DL2/CpG ODN interaction mediates Sezary syndrome malignant T cell apoptosis[J]. J Invest Dermatol, 2015, 135(1): 229–237. |
| [17] | 杨锋, 孙玉华, 刘佃滨, 等. 骨质疏松患者骨碱性磷酸酶、钙、磷代谢变化及与牙槽骨骨密度的相关性[J]. 中国骨质疏松志, 2017, 23(9): 1160–1166. |
| [18] | 周岳, 申玉芹, 高涵, 等. MT01对Pg感染的成骨细胞MG63特异性成骨相关因子表达的影响[J]. 口腔医学研究, 2016, 32(1): 1–4. DOI:10.3877/cma.j.issn.1674-1366.2016.01.001 |
| [19] | ZHENG Y, LIN C T, HOU X, et al. Enhancing the osteogenic capacity of MG63 cells through N-isopropylacrylamide-modified polyethylenimine-mediated oligodeoxynucleotide MT01 delivery[J]. RSC Adv, 2017, 7(43): 27121–27127. DOI:10.1039/C6RA27182K |
| [20] | LIN T H, SATO T, BARCAY K R, et al. NF-κB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle[J]. Tissue Eng Part A, 2015, 21(5/6): 875–883. |
 2019, Vol. 45
2019, Vol. 45


