扩展功能
文章信息
- 陈敏, 谢明, 万娟
- CHEN Min, XIE Ming, WAN Juan
- 癫痫发作患者血清Nesfatin-1动态变化及其对评估病情和短期预后的临床价值
- Dynamic variety of serum Nesfatin-1 and its clinical values in evaluation on illness condition and short-term prognosis in patients with epileptic seizure
- 吉林大学学报(医学版), 2019, 45(01): 105-110
- Journal of Jilin University (Medicine Edition), 2019, 45(01): 105-110
- 10.13481/j.1671-587x.20190120
-
文章历史
- 收稿日期: 2018-01-23
近年来癫痫患病率呈逐年增加趋势,主要以反复的痫性发作为特征,严重影响患者的日常生活,其中癫痫持续状态(status epilepticus,SE)常因发作时间过长会造成不可逆的脑损伤,因此致残率和病死率很高[1]。目前临床常应用多种量表对SE患者的病情程度及预后进行评定,然而量表的及时性和准确性难以满足临床需求,因此如何准确简便地评估SE患者病情及短期预后,对于SE患者临床确定治疗方案及康复治疗方案极为重要[2]。近年来研究[3-5]显示:癫痫发作时患者血清某些神经肽,如新饱食分子蛋白1(Nesfatin-1)、瘦素(leptin)和胃饥饿素(Ghrelin)等水平会发生变化,而这些神经肽的血清水平变化会影响脑功能及代谢和神经元兴奋性,其中Nesfatin-1是关注度较高的神经肽[6]。Nesfatin-1是一类具有调节能量平衡和摄食功能的肽类激素,越来越多的研究证实其参与并影响记忆、癫痫和睡眠等多种脑功能,已引起临床广泛的关注[7]。已有研究[8]证实:Nesfatin-1与SE患者预后结局有关联,但SE患者血清中Nesfatin-1水平的动态变化趋势以及血清Nesfatin-1水平能否有效评估SE患者的病情程度及短期预后等问题临床尚无明确报道。本研究以本院住院SE患者为研究对象,通过动态监测其血清Nesfatin-1水平,评估血清Nesfatin-1水平与SE患者病情及短期预后的相关性,探讨血清Nesfatin-1水平对癫痫发作患者预后判断的应用价值。
1 资料与方法 1.1 病例选择选取2015年6月—2016年10月本院住院SE患者43例,其中男性18例,女性25例,平均年龄(47.5±13.6)岁,其中部分性发作继发全面性发作(SGE)24例,原发性全面性发作(PGE)19例。患者及家属均书面签订知情同意书。纳入标准:①SE诊断标准均符合2001年国际抗癫痫联盟制定的癫痫诊断标准[9];②研究对象出院后均依医嘱门诊随访,相关资料齐备。排除标准:①诊断为颅脑外伤、脑卒中或颅内感染等疾病继发癫痫;②罹患心、肺、肝和肾等严重脏器功能不全;③存在严重电解质酸碱失衡状态。本研究经本院伦理委员会审核通过。抗癫痫治疗方法:诊断为SGE患者给予卡马西平片口服治疗,起始剂量0.1g,维持剂量0.2g;诊断为PGE患者给予丙戊酸钠片口服治疗,起始剂量为10 mg·kg-1,维持剂量为10 mg·kg-1·d-1。
1.2 患者血清Nesfatin-1水平检测所有SE患者均检测血清Nesfatin-1水平,检测时间分别为治疗前和治疗第1周末、第2周末和第1月末。本研究纳入的SE患者均在入院第2天清晨空腹抽取2~5mL外周静脉血,分离血清后,应用酶联免疫吸附法(ELISA法)测定血清样本中Nesfatin-1水平。检测仪器为全自动酶免疫分析仪(UniCel DxI 800,美国Beckman Coulter公司),相应ELISA检测试剂盒购自美国ROCH公司,按照试剂盒说明书进行检测,最后在450nm波长处测量吸光度(A)值,计算血清样本Nesfatin-1水平。
1.3 癫痫病情程度评估本研究采用利物浦痫性发作严重程度量表 2.0(LSSS2.0)[10]对患者本人及家属进行结构性访谈,主要根据12个指标来评估患者SE严重程度,包括:SE自觉严重程度、失去知觉时间、发作时行为异常、发作时皮肤潮湿情况、发作时摔倒情况和发作时咬舌情况;发作时其他受伤情况、发作后慌乱感、慌乱感持续时间、发作后头痛、发作后困倦和发作后状态持续时间等。原始LSSS评分为上述所有12个指标的评分之和,最低分为0分,最高分为40分;校正LSSS评分=原始LSSS评分/40×100,校正后LSSS评分最低为0分,最高为100分,校正后LSSS评分越高说明SE病情程度越严重。
1.4 分组所有患者出院后按照要求接受门诊或电话随访,无失访患者。随访时间7~18个月,平均随访时间(12.6±6.3)个月。根据最后一次随访患者的生存情况分为生存组(31例)和死亡组(12例)。其中10例患者因癫痫发作导致呼吸循环衰竭死亡,2例患者因癫痫发作导致意外死亡。2组患者年龄、性别构成比、病程和脑卒中等既往病史比较差异无统计学意义(P>0.05)。
1.5 统计学分析采用SPSS 21.0统计软件进行统计学分析。不同时间点患者LSSS评分和血清Nesfatin-1水平以x±s表示,同一时间点2组间样本均数比较采用成组t检验,同组不同时间点样本均数比较采用单因素方差分析,每2个时间点样本均数比较采用SNK-q检验。各时间点血清Nesfatin-1水平与LSSS评分的相关性分析采用Pearson直线回归。随访1年预后不良相关的危险因素应用多元Logistic回归分析。血清Nesfatin-1水平对SE患者预后的诊断价值采用受试者工作特征(ROC)曲线判断。以P < 0.05为差异有统计学意义。
2 结果 2.1 2组SE患者各时间点LSSS评分及预后结局经过正规抗癫痫治疗后,随访期间患者死亡12例,随访期间发作次数为(3.4±1.0)次,再次发作时间为(28.6±5.4)min。治疗前至治疗第1个月末,2组SE患者LSSS评分均呈进行性降低(P < 0.05)。治疗前2组SE患者LSSS评分比较差异无统计学意义(P>0.05)。治疗后第1周末至第1个月末,生存组SE患者LSSS评分均低于死亡组(P < 0.01)。见表 1。
| (x±s) | |||||||
| Group | n | LSSS score | F | P | |||
| Beforetreatment | End of 1st week after treatment | End of 2nd week after treatment | End of 1st month after treatment | ||||
| Survival | 31 | 44.1±5.8 | 33.5±4.6 | 28.7±4.2 | 21.3±3.8 | 10.375 | 0.018 |
| Death | 12 | 47.9±6.6 | 40.7±5.9 | 35.2±5.4 | 33.9±4.5 | 9.107 | 0.036 |
| t | 1.855 | 4.251 | 4.199 | 9.265 | - | - | |
| P | 0.071 | < 0.01 | < 0.01 | < 0.01 | - | - | |
| “-”:No data. | |||||||
治疗前至治疗第1个月末,2组SE患者血清Nesfatin-1水平均呈进行性降低(P < 0.05)。治疗前2组SE患者血清Nesfatin-1水平比较差异无统计学意义(P>0.05);治疗后第1周末至第1个月末,生存组SE患者血清Nesfatin-1水平均低于死亡组(P < 0.05)。见表 2。
| [x±s,ρB/(μg·L-1)] | |||||||
| Group | n | Nesfatin-1 | F | P | |||
| Before treatment | End of 1st week after treatment | End of 2nd week after treatment | End of 1st month after treatment | ||||
| Survival | 31 | 2.8±0.8 | 2.4±0.5 | 1.9±0.6 | 1.2±0.4 | 13.021 | 0.002 |
| Death | 12 | 3.3±0.9 | 3.0±0.7 | 2.5±0.8 | 1.9±0.6 | 11.343 | 0.010 |
| t | 1.776 | 3.147 | 2.675 | 4.454 | - | - | |
| P | 0.083 | < 0.05 | < 0.05 | < 0.05 | - | - | |
| “-”:No data. | |||||||
在4个时间点分别进行血清Nesfatin-1水平与LSSS评分的Pearson直线回归分析,结果显示:各个时间点2组SE患者血清Nesfatin-1水平与LSSS评分均呈正相关关系(r=0.617~0.726, P < 0.05)。见表 3。
| Group | Before treatment | End of 1st week after treatment | End of 2nd week after treatment | End of 1st month after treatment |
| Survival | ||||
| r | 0.617 | 0.695 | 0.726 | 0.707 |
| P | 0.044 | 0.033 | 0.024 | 0.029 |
| Death | ||||
| r | 0.673 | 0.731 | 0.718 | 0.644 |
| P | 0.035 | 0.023 | 0.026 | 0.040 |
以患者年龄、性别、病程和血清Nesfatin-1水平为自变量,以随访1年预后为因变量(0=生存,1=死亡),进行多元Logistic回归分析,结果显示:血清高水平Nesfatin-1(β=1.096,P=0.026)是SE患者随访1年预后不良的危险因素,但患者年龄、性别和病程均与随访1年预后不良无明显相关性(P>0.05)。见表 4。
| Clinical parameter | Regression coefficient (β) | Standard error (S.E.) | Wald coefficient | OR | P |
| Age | 0.129 | 0.117 | 1.221 | 1.138 | 0.101 |
| Gender | 0.089 | 0.309 | 0.083 | 1.093 | 0.146 |
| Course | 0.505 | 0.425 | 1.412 | 1.657 | 0.087 |
| Level of serum Nesfatin-1 | 1.096 | 0.416 | 6.945 | 2.993 | 0.026 |
血清Nesfatin-1水平≤2.7μg·L-1时,治疗前血清Nesfatin-1水平预测SE患者随访1年预后不良发生的曲线下面积(AUC)最大,AUC为0.862(P=0.026),且诊断灵敏度为88.5%,诊断特异度为79.6%。见图 1。
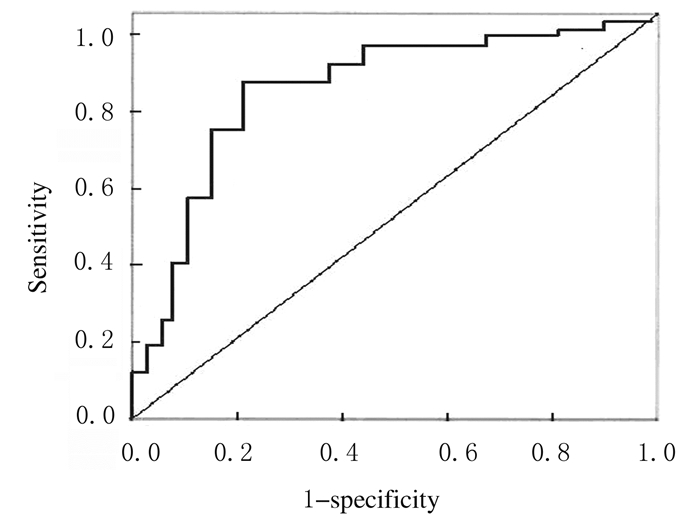
|
| 图 1 治疗前SE患者血清Nesfatin-1水平预测随访1年预后不良的ROC曲线 Fig. 1 ROC curves of levels of serumNesfatin-1 before treatment inpredicting poor prognosis after one year follow-up inSE patients |
|
|
癫痫是常见的神经系统疾病,其具体发病机制仍未完全明确,近年来神经肽在癫痫的发病中作用成为研究的热点之一[1]。已有研究[11-13]证实:癫痫发作时神经肽激素的分泌会发生明显变化,血清神经肽激素动态变化可能会影响大脑神经电活动的兴奋性,进而诱导癫痫发作。目前临床已开始对诸多神经肽进行深入研究,其中Nesfatin-1是新近在脑干和下丘脑中发现的分泌肽,也是较受关注的神经肽[14-15]。Nesfatin-1是核蛋白2经激素原转化酶剪切的一个片段,主要由下丘脑室旁核、视上核、弓状核、孤束核及外侧区产生,生理作用是抑制摄食功能,同时亦具有一定调节精神反应的作用[16]。实验动物研究[17]显示:Nesfatin-1可刺激室旁核和弓状核神经元发生去极化和超极化,且Nesfatin-1给药越多,神经元兴奋性越强,这一机制可能与大脑癫痫发作有关。近年来国外临床研究[18-19]显示:癫痫发作患者的血清和唾液中Nesfatin-1水平明显升高,治疗后血清Nesfatin-1水平逐步下降,但仍高于正常人群。因此目前临床认为Nesfatin-1与癫痫发作存在密切关联,然而血清Nesfatin-1水平的动态变化对于评估癫痫发作患者脑损伤以及短期预后的临床价值,仍未见临床报道,故本研究通过分析本院诊治的癫痫发作患者的临床资料对此进行探讨。
癫痫的病情严重程度主要是由其脑损伤程度决定的,评估其病情严重程度应包括发作过程中及发作后的多项指标。LSSS2.0量表可定量评价SE患者对自身癫痫严重程度的感受,采用LSSS评分评估癫痫严重程度可靠而灵敏,因此本研究选择LSSS评分作为癫痫发作病情严重程度的指标[10]。本研究结果证实:给予SE患者有效的临床治疗,其血清Nesfatin-1水平逐渐降低;同时结果也显示:尽管2组SE患者的临床治疗方案和疗程均无差异,但随访1年生存组SE患者血清Nesfatin-1水平仍明显低于死亡组患者,且降低程度也较大,提示预后结局不同的SE患者血清Nesfatin-1水平及动态变化趋势也不同,此结论与既往国外的研究相符[20]。国外研究[21]证实:癫痫患者血清Nesfatin-1水平明显增高,且在抗癫痫治疗后,Nesfatin-1水平下降,认为血清Nesfatin-1水平可作为癫痫发作预测和药物治疗效果判断的生物标记物。
本研究结果显示:SE患者血清Nesfatin-1水平与LSSS评分呈正相关关系,而LSSS评分是目前临床公认的反映SE患者病情严重程度较好的指标,提示血清Nesfatin-1水平能较好反映SE患者病情,且血清Nesfatin-1水平越高,SE患者病情越严重。进一步通过多元Logistic回归分析显示:血清高水平Nesfatin-1是随访1年SE患者预后不良的危险因素,因此可通过检测血清Nesfatin-1水平预测SE患者短期预后结局。为进一步分析其预测价值,本研究选择治疗前血清Nesfatin-1水平来预测SE患者随访1年死亡的可能性,ROC曲线分析显示:以2.7μg·L-1为切点时AUC最大,此时血清Nesfatin-1水平评估SE患者随访1年死亡的敏感度和特异度均大于75%,证实血清Nesfatin-1水平高于2.7μg·L-1的SE患者临床上应高度重视,将其作为高危人群早期予以干预治疗。
综上所述,本研究证实血清Nesfatin-1水平能有效评估SE患者的病情严重程度以及随访1年的预后结局,在临床治疗中应动态监测SE患者血清Nesfatin-1水平,并以此来评估SE患者临床疗效以及短期预后。然而本研究对于SE患者血清Nesfatin-1水平增高的具体机制尚未进行深入探讨,并且是否存在Nesfatin-1水平升高与癫痫发作互为因果关系也存在争议,因此有待进一步研究。
| [1] | 夏敏, 刘栋, 王艳玲, 等. 成人原发性癫痫患者血清酰基化ghrelin和nesfatin-1水平变化的意义[J]. 中华诊断学电子杂志, 2017, 5(2): 120–123. |
| [2] | WU G Q, CHOU X M, JI W J, et al. The prognostic value of plasma nesfatin-1 concentrations in patients with traumatic brain injury[J]. Clin Chim Acta, 2016, 458: 124–128. DOI:10.1016/j.cca.2016.05.001 |
| [3] | GE T, YANG W, FAN J, et al. Preclinical evidence of ghrelin as a therapeutic target in epilepsy[J]. Oncotarget, 2017, 8(35): 59929–59939. |
| [4] | SARAC M, BAKAL U, KULOGLU T, et al. Effects of carnosine and vitamin E on nucleobindin 2(NUCB2)/nesfatin-1, ghrelin, adropin, and irisin in experimentally induced ovarian torsion[J]. Ann Clin Lab Sci, 2018, 48(3): 345–354. |
| [5] | ALGUL S, OZCELIK O. Evaluating the levels of Nesfatin-1 and Ghrelin hormones in patients with moderate and severe major depressive disorders[J]. Psychiatry Investig, 2018, 15(2): 214–218. DOI:10.30773/pi.2017.05.24 |
| [6] | WEI Y, LI J, WANG H, et al. NUCB2/nesfatin-1:Expression and functions in the regulation of emotion and stress[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2018, 81: 221–227. DOI:10.1016/j.pnpbp.2017.09.024 |
| [7] | ENGSTER K M, KROCZEK A L, ROSE M, et al. Peripheral injection of bombesin induces c-Fos in NUCB2/nesfatin-1 neurons[J]. Brain Res, 2016, 1648(Pt A): 46–53. |
| [8] | PAŁASZ A, KRZYSTANEK M, WORTHINGTON J, et al. Nesfatin-1, a unique regulatory neuropeptide of the brain[J]. Neuropeptides, 2012, 46(3): 105–112. |
| [9] | BLUME W T, LÜDERS H O, MIZRAHI E, et al. Glossary of descriptive terminology for ictal semiology:Report of the ILAE task force on classification and terminology[J]. Epilepsia, 2001, 42(9): 1212–1218. |
| [10] | 冯海燕, 张宁, 李筱瑜, 等. 利物浦痫性发作严重程度量表评价左乙拉西坦添加治疗成人癫痫疗效的临床对照研究[J]. 癫痫与神经电生理学杂志, 2015, 24(1): 22–25. |
| [11] | PAŁASZ A, JANAS-KOZIK M, BORROW A, et al. The potential role of the novel hypothalamic neuropeptides nesfatin-1, phoenixin, spexin and kisspeptin in the pathogenesis of anxiety and anorexia nervosa[J]. Neurochem Int, 2018, 113: 120–136. DOI:10.1016/j.neuint.2017.12.006 |
| [12] | KORUCU CÇ, ATAYI M, ZAYIF S S, et al. May nesfatin-1 be a state marker in major depressive disorder with suicidal ideation[J]. Psychiatry Res, 2018, 267: 272–276. DOI:10.1016/j.psychres.2018.05.086 |
| [13] | XIAO M M, LI J B, JIANG L L, et al. Plasma nesfatin-1 level is associated with severity of depression in Chinese depressive patients[J]. BMC Psychiatry, 2018, 18(1): 88–91. DOI:10.1186/s12888-018-1672-4 |
| [14] | WEIBERT E, STENGEL A. The X/A-like cell revisited-spotlight on the peripheral effects of NUCB2/nesfatin-1 and ghrelin[J]. J Physiol Pharmacol, 2017, 68(4): 497–520. |
| [15] | ÜNAL K, YÜKSEL R N, TURHAN T, et al. The association of serum nesfatin-1 and ghrelin levels with metabolic syndrome in patients with schizophrenia[J]. Psychiatry Res, 2018, 261: 45–49. DOI:10.1016/j.psychres.2017.12.041 |
| [16] | PAŁASZ A, ROJCZYK E, BOGUS K, et al. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study[J]. Neurosci Lett, 2015, 592: 17–21. DOI:10.1016/j.neulet.2015.02.060 |
| [17] | SCHARNER S, PRINZ P, GOEBEL-STENGEL M, et al. Activity-based anorexia activates nesfatin-1 immunoreactive neurons in distinct brain nuclei of female rats[J]. Brain Res, 2017, 1677: 33–46. DOI:10.1016/j.brainres.2017.09.024 |
| [18] | SAHPOLAT M, ARI M. Plasma nesfatin 1 level in patients with first attack psychosis[J]. Bratisl Lek Listy, 2017, 118(2): 77–79. |
| [19] | DORE R, LEVATA L, LEHNERT H, et al. Nesfatin-1:functions and physiology of a novel regulatory peptide[J]. J Endocrinol, 2017, 232(1): R45–R65. DOI:10.1530/JOE-16-0361 |
| [20] | CAKIR M, CALIKOGLU C, YILMAZ A, et al. Serum nesfatin-1 levels:a potential new biomarker in patients with subarachnoid hemorrhage[J]. Int J Neurosci, 2017, 127(2): 154–160. DOI:10.3109/00207454.2016.1153473 |
| [21] | AYDIN S, DAG E, OZKAN Y, et al. Time-dependent changes in the serum levels of prolactin, nesfatin-1 and ghrelin as a marker of epileptic attacks young male patients[J]. Peptides, 2011, 32(6): 1276–1280. DOI:10.1016/j.peptides.2011.04.021 |
 2019, Vol. 45
2019, Vol. 45


