扩展功能
文章信息
- 江仁, 冯智英, 李平, 李红, 李双月
- JIANG Ren, FENG Zhiying, LI Ping, LI Hong, LI Shuangyue
- 坐骨神经脉冲射频对慢性坐骨神经压迫损伤模型大鼠脊髓背角胶质细胞活化水平的影响及其镇痛作用
- Effect of sciatic nervepulsed radiofrequency glial activation levels in spinal dorsal horn in chronic constriction injury rat models and its analgesia effect
- 吉林大学学报(医学版), 2019, 45(01): 45-50
- Journal of Jilin University (Medicine Edition), 2019, 45(01): 45-50
- 10.13481/j.1671-587x.20190109
-
文章历史
- 收稿日期: 2018-09-15
2. 浙江省宁波市鄞州区第二医院麻醉科, 浙江 宁波 315100
2. Department of Anesthesiology, Yinzhou Second Hospitalof Ningbo City, Zhejiang Province, Ningbo 315100, China
神经病理性疼痛(neuropathic pain, NP)是指外周或中枢神经系统原发和(或)继发性损害、功能障碍或短暂性紊乱引起的疼痛[1], 以自发性疼痛、痛觉过敏和痛觉超敏为特征,常导致患者生活质量明显降低。NP发病机制不仅涉及神经损伤和兴奋性刺激,还包括神经毒性以及免疫功能失调等,近年来研究[2]显示:活化的胶质细胞可影响并调节神经元的兴奋性,在神经元细胞和胶质细胞之间建立双向信号传递系统,进而诱导NP的产生和发展。抑制胶质细胞活化水平进而阻断进一步的级联反应将在临床疼痛治疗和镇痛药物研发中发挥重要作用。
大量临床资料[3-5]表明:背根神经节(dorsal root ganglion,DRG)脉冲射频(pulsed radiofrequency, PRF)能有效缓解多种NP,如带状疱疹后神经痛、颈神经根性疼痛和腰腿痛等。但对于基层医院,医生临床经验和影像设备不足,限制了DRG行PRF的开展。外周神经PRF直接作用于外周神经治疗多种NP同样有效[6],安全且操作简单,对影像设备要求低,更适合基层医院开展。至今,有关外周神经PRF镇痛机制的研究甚少。本研究建立慢性坐骨神经压迫损伤(chronic constriction injury, CCI)模型,在坐骨神经结扎近端行标准PRF以探讨其作用机制,为PRF的临床应用提供理论依据。
1 材料与方法 1.1 主要试剂、仪器和实验动物及分组小胶质细胞特异性蛋白(Iba-1)、胶质原纤维酸性蛋白(GFAP)一抗和羊抗兔IgG抗体(美国Protein Technology公司),ECL发光试剂盒(美国Thermo公司)。凝胶成像分析仪(美国BIO-RAD)。SPF级雄性SD大鼠40只,体质量180~ 200 g,由浙江大学实验动物中心提供, 动物合格证号:SYXK(浙)2014-0008。大鼠自由摄食饮水,饲养温度为(25±1)℃,分笼饲养。所有与动物相关的实验均经浙江大学伦理委员会审核通过。本实验过程严格按照国际疼痛研究协会(IASP)关于使用动物进行疼痛实验研究纲要要求实施完成。采用随机数字法将大鼠分4组,每组10只。SS组:CCI假造模加PRF假治疗组,仅分离坐骨神经不结扎不行PRF治疗;SP组:CCI假造模加PRF治疗组,仅分离坐骨神经不结扎行PRF治疗;CS组:CCI造模加PRF假治疗组,分离结扎坐骨神经不行PRF治疗;CS组:CCI造模加PRF治疗组,分离结扎坐骨神经行PRF治疗。
1.2 CCI模型制备SD大鼠采用10%水合氯醛(400mg·kg-1)腹腔注射麻醉,切开皮肤(约2cm)并钝性分离暴露坐骨神经干,4.0铬制肠线环绕神经干做4个结扎环,间距1mm,以不完全阻断血管为度,打结时可见肢体轻微抽动。缝合皮肤切口,消毒,皮下注射100 000 U青霉素。出现足外翻、跛行、脚趾弯曲聚拢和行走时抬足等行为学变化表示造模成功。假手术组大鼠仅接受分离暴露坐骨神经,其他步骤相同。
1.3 PRF治疗CCI造模后4d,使用脉冲射频仪(PMG-230, 加拿大Baylis公司)、电极针(22G, SMK-10, 美国Radionics公司)作用于大鼠坐骨神经结扎近端(0.2~0.3cm)。消毒铺巾后,SP组和CP组大鼠行PRF(120s, 42℃);SS组和CS组大鼠以相同方法相同位置放置射频电极针,无脉冲电流。
1.4 疼痛行为学测定各组大鼠于CCI造模前1d(D0)及造模后1、3、5和7d (D1、D3、D5和D7)进行疼痛行为学测定。所有测定均由固定人员于固定时间(8:00~12:00, AM)在安静环境下完成。①机械缩足反射阈值(mechanical withdrawal threshold, MWT)测定:利用von Frey电子测痛仪测量大鼠患侧后足机械疼痛阈值。将大鼠放置于金属笼内,适应30min待大鼠处于安静状态,von Frey电子测痛仪针尖垂直刺激大鼠右后足第三、四足底皮肤,出现快速缩足反应或舔足动作记录为大鼠MWT。②热缩足反射潜伏期(thermal paw withdrawal latency, TWL)测定:按Hargreaves法用热辐射刺激仪照射大鼠足底。热辐射刺激仪(10 V, 50 W; 浙江大学研制)光斑直径为0.8 cm。照射大鼠足底中后1/3处,出现回缩、舔足、抬足和撕咬等反应的时间作为大鼠TWL。间隔3 min测定1次,切断时间为20s。
1.5 Western blotting法检测大鼠患侧脊髓背角Iba-1和GFAP蛋白表达水平疼痛行为学完成后(D7),大鼠经10%水合氯醛(400 mg·kg-1)腹腔注射麻醉,快速断头,冰浴中快速取患侧L3~5节段脊髓背角, 液氮保存。冰浴匀浆,加入含蛋白酶和磷酸酶抑制剂的SDS缓冲液(每毫克组织10mL),采用BCA法测定蛋白浓度后样品沸水浴5 min,等量蛋白样品10%SDS-聚丙烯酰胺凝胶电泳,应用湿转膜法,将蛋白转移到PVDF膜上。用5%脱脂牛奶封闭60 min,在4℃条件下分别用一抗Iba-1(1:200)、GFAP (1:2000)摇床孵育过夜。羊抗兔IgG抗体(1:2000)室温孵育90 min。再次洗膜后,用ECL发光试剂盒,在凝胶成像分析仪处理系统中成像。用Iamge Lab软件分析条带的平均灰度值,代表Iba-1和GFAP蛋白表达水平。
1.6 统计学分析采用SPSS17.0统计软件进行统计学分析。大鼠MWT和TWL及脊髓背角Iba-1和GFAP蛋白表达水平均符合正态分布,以x±s表示,MWT和TWL组间比较采用重复测量方差分析,Iba-1和GFAP表达水平组间比较采用单因素方差分析,组间两两比较采用SNK-q检验。以P < 0.05表示差异有统计学意义。
2 结果 2.1 各组大鼠疼痛行为学检测结果各组大鼠MWT和TWL基础值(D0)比较差异无统计学意义(P > 0.05);与SS组比较,CS组和CP组大鼠CCI造模后不同时间点患侧出现足外翻、跛行、脚趾弯曲聚拢和行走时抬足等行为学变化,MWT和TWL均明显下降(P < 0.01);与CS组比较,CP组大鼠(D5和D7)PRF治疗后,足外翻、跛行、脚趾弯曲聚拢和行走时抬足等行为学变化明显缓解,MWT和TWL值明显升高(P < 0.01)。见表 1。
| (n=10, x±s) | ||||||||||
| Group | MWL(F/N) | TWL(t/s) | ||||||||
| D0 | D1 | D3 | D5 | D7 | D0 | D1 | D3 | D5 | D7 | |
| SS | 63.1±5.5 | 60.0±3.4 | 64.7±2.3 | 60.4±4.2 | 64.5±2.6 | 19.4±0.9 | 19.2±0.6 | 19.0±1.1 | 19.4±0.7 | 19.5±0.6 |
| SP | 64.9±4.1 | 59.6±4.2 | 59.3±8.0 | 57.9±8.4 | 58.7±6.7 | 19.5±0.6 | 19.0±0.9 | 18.6±1.3 | 19.1±1.0 | 18.7±0.9 |
| CS | 64.5±3.8 | 29.8±3.6* | 27.1±5.2* | 22.6±4.2* | 23.5±6.7* | 19.9±0.1 | 16.6±1.4* | 12.8±1.9* | 9.6±2.0* | 8.1±1.4* |
| CP | 59.6±6.4 | 27.4±4.7* | 26.3±5.5* | 37.1±3.4*△ | 35.6±3.4*△ | 19.7±0.8 | 14.8±1.7* | 12.7±1.9* | 14.5±2.4*△ | 13.8±1.4*△ |
| SS group:CCI sham-operation+RPF sham group; SP group: CCI sham-operation+RPF group; CS group:CCI+RPF sham group; CP group:CCI+RPF group. *P < 0.01 compared with SS group;△P < 0.01 compared with CS group. | ||||||||||
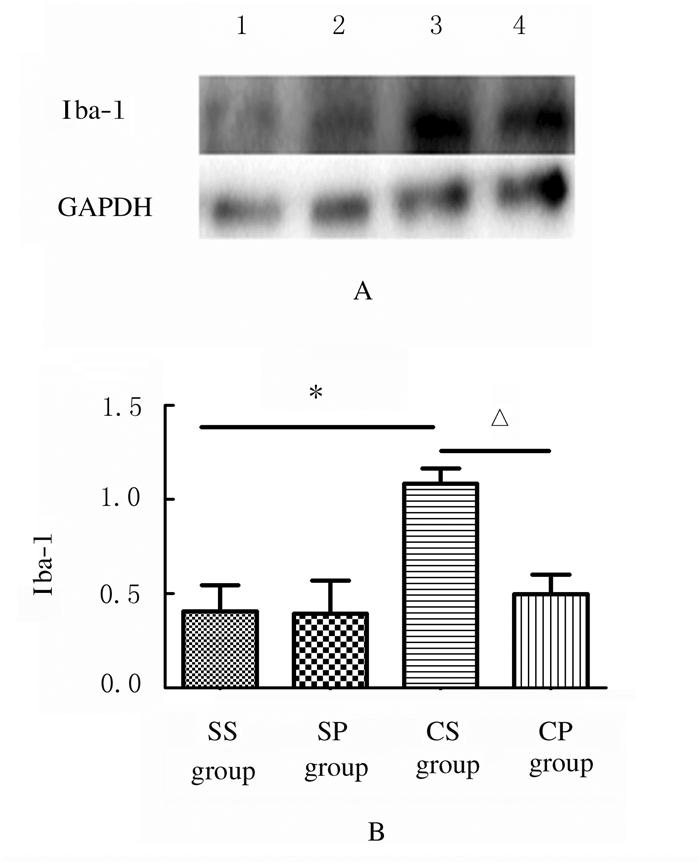
与SS组比较,CS组大鼠脊髓背角Iba-1蛋白表达水平明显升高(t=4.194, P < 0.05);与CS组比较,CP组大鼠脊髓背角Iba-1蛋白表达水平明显降低(t=4.491, P < 0.05)。见图 1。
|
| SS group:CCI sham-operation+RPF sham group; SP group: CCI sham-operation+RPF group; CS group:CCI+RPF sham group; CP group:CCI+RPF group.Lane 1: SS group; Lane 2: SP group; Lane 3: CS group; Lane 4: CP group. *P < 0.05 compared with SS group; △P < 0.05 compared with CS group. 图 1 Western blotting法检测各组大鼠脊髓背角Iba-1蛋白表达电泳图(A)和直条图(B) Fig. 1 Electrophoregram (A) and histogram (B) of expressions of Iba-1 protein in spinal dorsal horn of rats in various groups detected by Western blotting method |
|
|
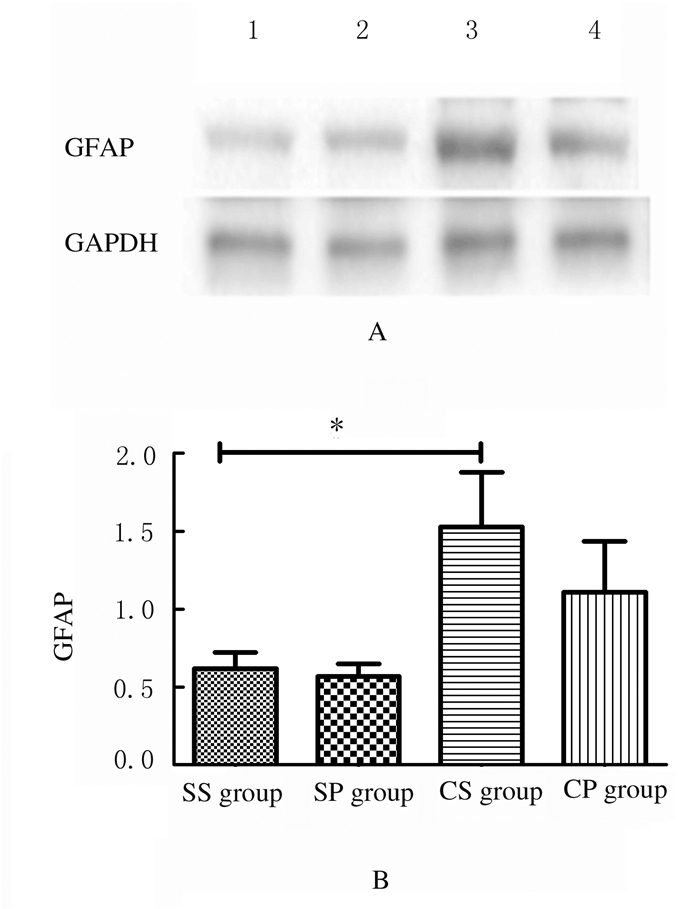
与SS组比较,CS组大鼠脊髓背角GFAP蛋白表达水平明显升高(t=2.497, P < 0.05);与CS组比较,CP组大鼠脊髓背角GFAP蛋白表达水平差异无统计学意义(t=0.878, P > 0.05)。见图 2。
|
| SS group:CCI sham-operation+RPF sham group; SP group: CCI sham-operation+RPF group; CS group:CCI+RPF sham group; CP group:CCI+RPF group.Lane 1: SS group; Lane 2: SP group; Lane 3: CS group; Lane 4: CP group.*P < 0.05 compared with SS group. 图 2 Western blotting法检测各组大鼠脊髓背角GFAP蛋白表达电泳图(A)和直条图(B) Fig. 2 Electrophoregram (A) and histogram (B) of expressions of GFAP protein in spinal dorsal horn of rats in various groups detected by Western blotting method |
|
|
1997年PRF首次应用于临床疼痛治疗,与传统连续射频比较,其最高温度不超过42℃,产生不依赖蛋白凝固变性的镇痛效果,治疗后不出现感觉减退及运动神经损伤等异常表现[7]。PRF治疗多种NP效果明显且不良反应少,一经提出迅速被患者和医疗人员接受并广泛开展,但其镇痛机制至今尚未阐明。本研究结果提示:坐骨神经PRF在改善CCI模型大鼠疼痛行为学同时抑制了脊髓背角小胶质细胞活化,表明坐骨神经PRF能有效镇痛,其机制可能与抑制脊髓背角小胶质细胞活化有关。
PRF根据靶点分中枢神经PRF和外周神经PRF,中枢神经PRF主要以DRG为靶点,而外周神经PRF则可选择坐骨神经、肩胛上神经和腓肠神经等为靶点。外周神经PRF镇痛效果确切,临床上已开展坐骨神经PRF治疗复杂性区域疼痛综合征[8]、肩胛上神经PRF治疗慢性脑卒中后肩痛[9]和腓肠神经PRF治疗腓肠神经痛[10]等。本研究中,坐骨神经PRF后,CP组大鼠(D5和D7)足外翻和跛行等NP症状明显缓解,MWT和TWL明显升高。JIA等[11]将PRF直接作用于CCI模型坐骨神经干,治疗组大鼠MWT和TWL明显改善。LI等[12]发现:坐骨神经结扎处PRF可有效缓解CCI模型诱发的NP,加速靶神经超微结构恢复。本研究结果与上述研究结果一致,再次证实外周神经PRF可以有效缓解NP。与中枢神经PRF比较,外周神经PRF具有以下优点:①安全,不限制凝血功能障碍等患者;②简单,更容易被患者接受;③对影像设备要求低,更适合基层医院广泛开展。
目前文献研究多以DRG为靶点探讨PRF镇痛机制,而以外周神经为靶点研究探讨PRF机制的研究甚少。PRF镇痛机制涉及以下几个方面:①电场机制,高强度电磁场诱导跨膜电位改变离子通道和阈电位等进而影响信号传导[13];②激活内源性阿片肽通路[14];③抑制炎症介质相关信号通路[15];④与抑制脊髓背角胶质细胞活化有关;⑤加速受损神经修复。本研究以坐骨神经为靶点探索坐骨神经PRF镇痛机制是否与脊髓背角胶质细胞活化水平有关,结果显示:坐骨神经PRF抑制脊髓背角小胶质细胞活化,对星形胶质细胞活化无明显影响。星形胶质细胞主要起支持、营养和保护作用,活化后特异标志物GFAP表达增加,并释放大量活性介质。星形胶质细胞活化主要发生在疼痛发展和维持阶段,选择性敲除中枢神经系统编码GFAP蛋白基因或靶向给予星形胶质细胞抑制剂L-α-aminoadipate均能产生明显的镇痛效果[16]。本研究中,CCI造模后CS组大鼠MWT和TWL明显降低并伴随脊髓背角GFAP蛋白表达增加,提示星形胶质细胞参与NP进程;与CS组比较,坐骨神经PRF后CP组大鼠(D5和D7)MWT和TWL回升,而GFAP表达水平差异无统计学意义,表明坐骨神经PRF镇痛作用可能与脊髓背角星形胶质细胞无关。小胶质细胞是定居在中枢神经系统的免疫效应细胞,呈分支状,时时监测内环境变化。机械压迫或损伤、感染、肿瘤细胞浸润、代谢及营养性神经变化均能激活小胶质细胞,活化后演化为吞噬性小胶质细胞,表现为突起增大增粗,其特异性标志物(Iba-1和CD11b/OX-42等)表达水平明显增加,并释放多种活性介质,如脑源性神经营养因子(brain derived neurotrophic factor,BDNF)、一氧化氮、前列腺素、趋化因子和炎性细胞因子等。这些小胶质细胞源性介质均可调节神经元细胞兴奋性、突触功能活性以及疼痛敏化。小胶质细胞的活化始于疼痛的早期,介导多种疼痛的启动,应用小胶质细胞抑制剂Minocycline靶向药可阻断神经元细胞和胶质细胞间双向信号传递系统建立,推迟或缓解NP的进展[17]。PARK等[18]发现:脊神经结扎(SNL)模型疼痛敏化,伴随脊髓背角小胶质细胞活化,DRG行PRF治疗后,疼痛敏化缓解,同时伴随小胶质细胞活化水平下降。VALLEJO等[19]研究发现:CCI模型小胶质细胞活化并产生活性介质参与NP,DRG行PRF治疗明显缓解NP,抑制小胶质细胞活化。本研究结果显示:CS组大鼠CCI造模后MWT和TWL明显降低的同时Iba-1蛋白表达水平明显增加,坐骨神经PRF后CP组大鼠(D5和D7) MWT和TWL回升,Iba-1表达水平明显下调。及本研究结果:小胶质细胞与NP进程有密切关联,且无论是以DRG还是以坐骨神经为作用靶点,PRF均抑制脊髓背角小胶质细胞活化,进而缓解NP。
PRF抑制小胶质细胞活化途径研究报道甚少,可能与以下几方面有关:①PRF能有效遏制干扰素调节因子8(interferon regulatory factor 8, IRF8)表达,进而直接抑制小胶质细胞活化[20]。中枢神经系统中IRF8分布于小胶质细胞中,是启动活化小胶质细胞的关键介质,抑制IRF8的表达可阻断小胶质细胞的活化及其下游介质的释放,缓解NP进展[21]。②小胶质细胞活化释放一系列活性介质,通过突触、信使传递、基因转录和翻译等再次激活小胶质细胞,形成正反馈,引发NP瀑布式放大。PRF可通过调控活性介质的产生和释放[11, 22-24],阻断正反馈形成,抑制小胶质细胞进一步活化,缓解NP。③PRF加速靶神经超微结构恢复[11],避免伤害性刺激持续激活小胶质细胞。
本研究不足之处:未对DRG与坐骨神经PRF镇痛效果进行比较;外周神经PRF具体通过何种途径抑制脊髓背角小胶质细胞活化未进行深入研究,这将是本课题组以后研究的方向。
综上所述,脊髓背角小胶质细胞和星形胶质细胞活化与NP进程有密切关联,坐骨神经PRF能明显改善CCI模型大鼠疼痛敏化,其镇痛机制与抑制脊髓背角小胶质细胞活化水平有关,而与星形胶质细胞活化水平无明显关联。外周神经PRF的深入研究,有利于基层医院PRF推广应用,为更多NP患者提供安全而有效的治疗方法。
| [1] | HAANPAA M, ATTAL N, BACKONJA M, et al. NeuPSIG guidelines on neuropathic pain assessment[J]. Pain, 2011, 152(1): 14–27. DOI:10.1016/j.pain.2010.07.031 |
| [2] | LOGGIA M L, CHONDE D B, AKEJU O, et al. Evidence for brain glial activation in chronic pain patients[J]. Brain, 2015, 138(Pt 3): 604–615. |
| [3] | KIM K, JO D, KIM E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia[J]. Pain Physician, 2017, 20(3): E411–E418. |
| [4] | YOON Y M, HAN S R, LEE S J, et al. The efficacy of pulsed radiofrequency treatment of cervical radicular pain patients[J]. Korean J Spine, 2014, 11(3): 109–112. DOI:10.14245/kjs.2014.11.3.109 |
| [5] | SHANTHANNA H, CHAN P, MCCHESNEY J, et al. Pulsed radiofrequency treatment of the lumbar dorsal root ganglion in patients with chronic lumbar radicular pain:a randomized, placebo-controlled pilot study[J]. J Pain Res, 2014, 7: 47–55. |
| [6] | CHANG M C. Efficacy of pulsed radiofrequency stimulation in patients with peripheral neuropathic pain:A narrative review[J]. Pain Physician, 2018, 21(3): E225–E234. |
| [7] | SLUIJTER M E. Pulsed radiofrequency[J]. Anesthesiology, 2005, 103(6): 1313. |
| [8] | CHOI Y H, CHANG D J, HWANG W S, et al. Ultrasonography-guided pulsed radiofrequency of sciatic nerve for the treatment of complex regional pain syndrome type Ⅱ[J]. Saudi J Anaesth, 2017, 11(1): 83–85. DOI:10.4103/1658-354X.197366 |
| [9] | PICELLI A, LOBBA D, VENDRAMIN P, et al. A retrospective case series of ultrasound-guided suprascapular nerve pulsed radiofrequency treatment for hemiplegic shoulder pain in patients with chronic stroke[J]. J Pain Res, 2018, 11: 1115–1120. DOI:10.2147/JPR |
| [10] | ABD-EISAYED A, JACKSON M, Plovanich E. Pulsed radiofrequency ablation for treating sural neuralgia[J]. Ochsner J, 2018, 18(1): 88–90. |
| [11] | JIA Z, REN H, LI Q, et al. Pulsed radiofrequency reduced neuropathic pain behavior in rats associated with upregulation of GDNF expression[J]. Pain Physician, 2016, 19(2): 49–58. |
| [12] | LI D Y, MENG L, JI N, et al. Effect of pulsed radiofrequency on rat sciatic nerve chronic constriction injury:a preliminary study[J]. Chin Med J, 2015, 128(4): 540–544. DOI:10.4103/0366-6999.151113 |
| [13] | LIU Y, FENG Y, ZHANG T. Pulsed radiofrequency treatment enhances dorsal root ganglion expression of hyperpolarization-activated cyclic nucleotide-gated channels in a rat model of neuropathic pain[J]. J Mol Neurosci, 2015, 57(1): 97–105. DOI:10.1007/s12031-015-0582-x |
| [14] | WU B, NI J, ZHANG C, et al. Changes in spinal cord met-enkephalin levels and mechanical threshold values of pain after pulsed radio frequency in a spared nerve injury rat model[J]. Neurol Res, 2012, 34(4): 408–414. DOI:10.1179/1743132812Y.0000000026 |
| [15] | YEH C C, WU Z F, CHEN J C, et al. Association between extracellular signal-regulated kinase expression and the anti-allodynic effect in rats with spared nerve injury by applying immediate pulsed radiofrequency[J]. BMC Anesthesiol, 2015, 15: 92. DOI:10.1186/s12871-015-0071-3 |
| [16] | AUSTIN P J, MOALEM-TAYLOR G. The neuro-immune balance in neuropathic pain:involvement of inflammatory immune cells, immune-like glial cells and cytokines[J]. J Neuroimmunol, 2010, 229(1/2): 26–50. |
| [17] | SUN J S, YANG Y J, ZHANG Y Z, et al. Minocycline attenuates pain by inhibiting spinal microglia activation in diabetic rats[J]. Mol Med Rep, 2015, 12(2): 2677–2682. DOI:10.3892/mmr.2015.3735 |
| [18] | PARK H W, AHN S H, SON J Y, et al. Pulsed radiofrequency application reduced mechanical hypersensitivity and microglial expression in neuropathic pain model[J]. Pain Med, 2012, 13(9): 1227–1234. DOI:10.1111/j.1526-4637.2012.01453.x |
| [19] | VALLEJO R, TILLEY D M, VOGEL L, et al. The role of glia and the immune system in the development and maintenance of neuropathic pain[J]. Pain Pract, 2010, 10(3): 167–184. DOI:10.1111/(ISSN)1533-2500 |
| [20] | LIU R, XU X, XU Y, et al. Pulsed radiofrequency on dorsal root ganglion relieved neuropathic pain associated with downregulation of the spinal interferon regulatory factor 8, microglia, p38MAPK expression in a CCI rat model[J]. Pain Physician, 2018, 21(4): E307–E322. |
| [21] | AKAGI T, MATSUMURA Y, YASUI M, et al. Interferon regulatory factor 8 expressed in microglia contributes to tactile allodynia induced by repeated cold stress in rodents[J]. J Pharmacol Sci, 2014, 126(2): 172–176. DOI:10.1254/jphs.14143SC |
| [22] | LIN M L, LIN W T, HUANG R Y, et al. Pulsed radiofrequency inhibited activation of spinal mitogen-activated protein kinases and ameliorated early neuropathic pain in rats[J]. Eur J Pain, 2014, 18(5): 659–670. DOI:10.1002/ejp.2014.18.issue-5 |
| [23] | LEE J B, BYUN J H, CHOI I S, et al. The effect of pulsed radiofrequency applied to the peripheral nerve in chronic constriction injury rat model[J]. Ann Rehabil Med, 2015, 39(5): 667–675. DOI:10.5535/arm.2015.39.5.667 |
| [24] | 李坚, 李刚, 郭卫东, 等. TGN-020对大鼠脊髓损伤后继发性水肿和星形胶质细胞增生的影响[J]. 西安交通大学学报:医学版, 2018, 39(4): 685–690, 718. |
 2019, Vol. 45
2019, Vol. 45


