扩展功能
文章信息
- 徐金瑞, 吕翠萍, 杨易, 周彦兵, 骆佳, 王玉炯
- XU Jinrui, LYU Cuiping, YANG Yi, ZHOU Yanbing, LUO Jia, WANG Yujiong
- BCG感染牛肺泡巨噬细胞的转录组测序和分析
- Transcriptome sequencing and analysis of bovine alveolar macrophages infected with BCG
- 吉林大学学报(医学版), 2019, 45(01): 12-16
- Journal of Jilin University (Medicine Edition), 2019, 45(01): 12-16
- 10.13481/j.1671-587x.20190103
-
文章历史
- 收稿日期: 2018-05-24
结核病是由结核分枝杆菌(Mycobacterium tuberculosis, MTB)和牛分枝杆菌引起的慢性传染性疾病。WHO发布的2017年全球结核病报告显示:2016年全球结核病新发病例数为1 040万例,印度、印度尼西亚、中国、菲律宾和巴基斯坦等5个国家的结核病新发病例占56%[1]。据估计,约10%的人类结核病例是由牛分枝杆菌感染引起的[2]。因此,控制牛结核无疑是降低甚至免除人类感染牛结核风险的最佳措施。
MTB是典型的胞内寄生菌,肺泡巨噬细胞作为免疫调节细胞和效应细胞,在感染过程中通过吞噬、抗原提呈和分泌多种细胞因子等功能来调控机体炎症反应和免疫应答[3]。由于感染巨噬细胞的能力对细菌在宿主体内的传播和扩散至关重要,因此在抗结核免疫机制研究中,MTB与肺泡巨噬细胞的相互作用一直是研究的重点和热点。已有研究[4-6]表明:MTB感染后,可引起机体长链非编码RNA(long non-coding RNA,lncRNA)及mRNA表达谱表现异常,这些差异表达的lncRNAs参与调控Toll样受体(Toll like receptor,TLR)、转化生长因子β(transforming growth factor-β,TGF-β)及Hippo(HPO)等细胞信号通路。在结核分枝杆菌感染中,机体对病原的免疫学反应存在着种属特异性[7]。牛本身作为结核分枝杆菌感染的受害宿主,同时也是研究结核病理想的大型动物模型。因此,通过研究牛原代肺泡巨噬细胞与结核分枝杆菌间的相互作用,对阐明结核病的致病机制具有重要的意义。
本研究通过对卡介苗(Bacillus Calmette-Guérin,BCG)感染前后的牛肺泡巨噬细胞(bovine alveolar macrophage,BAM)进行转录组测序,并进行生物信息学分析,揭示差异表达的基因和lncRNA,为深入研究巨噬细胞抗MTB感染的免疫反应奠定基础。
1 材料与方法 1.1 材料BAM源于1.5岁西门达尔牛肺脏灌洗液;BCG购于成都生物制品研究所;DMEM培养基、胎牛血清和胰蛋白酶(美国Gibco公司),D-Hank’ s液(北京Biotopped公司),红细胞裂解液(北京TianGEN公司),Tween-80 (美国Amresco公司),Middlebrook 7H9肉汤基础和Middlebrook ADC增菌液(美国BD公司),青霉素和链霉素(美国Hyclone公司)。
1.2 实验分组及处理BCG感染12h后的BAM为感染组(B12),感染复数为10:1,以未感染的BAM为对照组(C12)。
1.3 检测方法① BCG培养:将BCG接种于含0.2% Tween-80和10%Middlebrook ADC增菌液的Middlebrook 7H9培养液中,置37℃、5%CO2培养箱中培养4~6周,测定吸光度[A(600)]值确定BCG浓度,A(600)=0.1时,相当于1×107个细菌[8];② BAM分离培养及BCG感染:在500 mL D-Hank’ s液中加入50 mg·L-1庆大霉素、2.5 mg·L-1两性霉素和100 mg·L-1双抗各1mL,灌洗牛肺,将灌洗液以2 000 r·min-1离心10 min后弃上清,用20 mL含10%胎牛血清、100 U·mL-1青霉素和100 U·mL-1链霉素的RPMI 1640培养液悬浮细胞,以80%的密度接种于直径15cm的培养皿中,细胞贴壁6~8h后换液,继续培养12~18h后消化、计数,以2×106mL-1密度接种于6孔板,贴壁16~18h,加入BCG悬液; ③样品制备:收集感染组和未感染组细胞至1.5mLEP管,加1mL Trizol于-80℃超低温冰箱保存;④转录组测序:将样品送至北京诺禾致源生物信息科技有限公司进行测序和数据分析。
1.4 分析指标及应用软件基因组比对采用Tophat (v2.0.9)软件,差异表达分析采用Cuffdiff(v1.3.0)软件,基因本体(Gene Ontology,GO)富集分析采用GOSeq软件(Release2.12),京都基因和基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)富集分析采用KOBAS(2.0)软件。
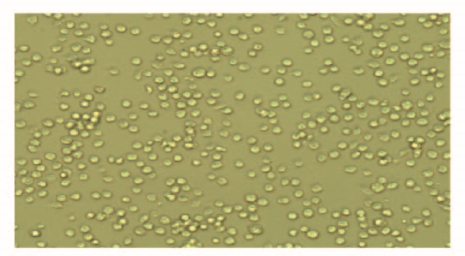
2 结果 2.1 BAM原代细胞培养结果显微镜下观察:分离培养的BAM形态为圆形或椭圆形,纯度达到95%以上(图 1,见插页一)。
|
| 图 1 原代培养BAM的形态表现(×200) Fig. 1 Morphology of primary BAM (×200) |
|
|
测序后,过滤后的测序数据(clean reads)感染组共计14.34 G,未感染组共计15.69 G (表 1)。99.00%碱基正确识别率(Q20):感染组为98.14%,未感染组为98.15%;99.90%碱基正确识别率(Q30):感染组为95.40%,未感染组为95.41%。测序质量能够满足后续分析的要求。
| Group | Raw reads | Clean reads | Clean bases | Error rate (η/%) | Q20 (η/%) | Q30 (η/%) | GC content (η/%) |
| B12 | 96598300 | 95594728 | 14.34G | 0.01 | 98.14 | 95.40 | 51.27 |
| C12 | 105600710 | 104633094 | 15.69G | 0.01 | 98.15 | 95.41 | 50.30 |
感染组和未感染组样品reads与参考基因组比对的总百分比(total mapped reads)分别为88.37%和89.14%,均高于70.00%,表明参考基因组选择合适。见表 2。
| Group | Total reads | Total mapped | Multiple mapped | Uniquely mapped | Read-1 | Read-2 | Reads map to “+” | Reads map to “-” | Non-splice reads | Splice reads | Reads mapped in proper pairs | Proper-paired reads map to different chrom |
| B12 | 95594728 | 84480060 (88.37%) | 8709599 (9.11%) | 75770461 (79.26%) | 39125889 (40.93%) | 36644572 (38.33%) | 37794724 (39.54%) | 37975737 (39.73%) | 51055094 (53.41%) | 24715367 (25.85%) | 68937428 (72.11%) | 10(0%) |
| C12 | 104633094 | 93265877 (89.14%) | 6681460 (6.39%) | 86584417 (82.75%) | 44782900 (42.80%) | 41801517 (39.95%) | 43171986 (41.26%) | 43412431 (41.49%) | 61021730 (58.32%) | 25562687 (24.43%) | 77949040 (74.50%) | 18(0%) |
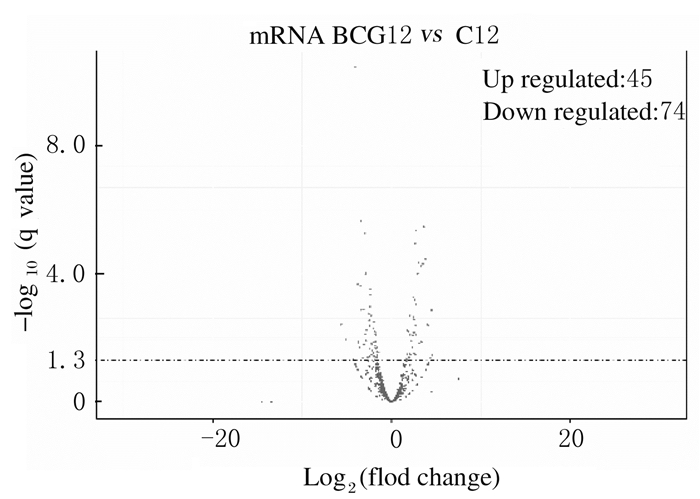
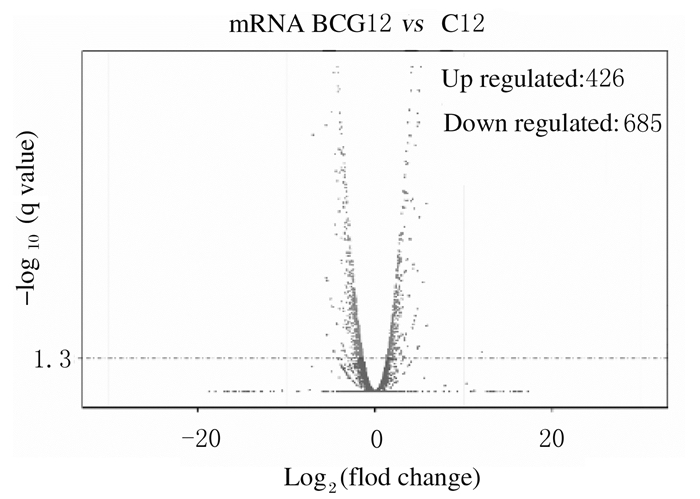
与未感染组比较,感染组差异表达的lncRNA共有119个(P < 0.05),其中表达上调45个,表达下调74个(图 2);差异表达的mRNA共有1 111个(P < 0.05),其中表达上调426个,表达下调685个(图 3)。
|
| 图 2 差异表达的lncRNA火山图 Fig. 2 Vocalno plot of differentially expressed lncRNA |
|
|
|
| 图 3 差异表达的mRNA火山图 Fig. 3 Vocalno plot of differentially expressed mRNA |
|
|
最显著富集项为参与免疫反应的GO:0006955(P < 0.05),涉及差异表达的基因共125个,其中促炎因子白细胞介素6(IL-6,Fold change=5.39)、白细胞介素7(IL-7,Fold change =3.12)和白细胞介素23A (IL-23A,Fold change=4.96)等63个基因表达上调,过氧化物酶体增殖剂激活受体γ(PPARγ,Fold change=-2.66)等62个基因表达下调(表 3),表明BCG感染BAM 12 h,激发了强烈的炎性反应。
| GO_accession | Description | Term_type | Candidate gene | Background gene |
| GO:0006955 | Immune response | Biological_process | 125 | 1 032 |
| GO:0002376 | Immune system process | Biological_process | 189 | 1 032 |
| GO:0005125 | Cytokine activity | Molecular_function | 41 | 1 032 |
对lncRNA位置相关性(co-location)靶基因进行KEGG pathway富集分析,最显著富集的通路为转化生长因子β(TGF-β)信号通路及ATP结合盒(ATP-bindingcassette,ABC)转运蛋白信号通路。见表 4。
| Term | Sample number | Background number | P value | KO | Entrez ID | Ensembl ID |
| TGF-β signaling pathway | 6 | 79 | 0.012 | bta:531391 bta:534579 bta:505453 bta:518880 bta:511077 bta:539315 |
531391 534579 505453 518880 511077 539315 |
MAPK, ERK1 TFDP1, CREBP, hmm291030, RBX1 MYC, CMYC ACR1B |
| ABC transporters | 4 | 42 | 0.019 | bta:504909 bta:537351 bta:510497 bta:100299651 |
504909 537351 510497 100299651 |
ABCA10 ABCA6 ABCA5 ABCA2 |
巨噬细胞是机体重要的免疫细胞,在宿主抗感染免疫中起关键作用[9]。巨噬细胞可为MTB提供营养物质和生存繁殖的场所,亦可通过细胞凋亡或自噬清除MTB[10]。MTB可阻止吞噬体酸化、吞噬体和溶酶体融合,以避免被蛋白水解酶水解和后续免疫应答事件的发生,是MTB逃避宿主细胞清除的主要策略[11-12]。但其具体机制和过程尚未阐明。
本研究对BCG感染后的BAM进行RNA-Seq及生物信息学分析,结果表明:在BCG感染后,BAM中lncRNA及mRNA表达谱发生改变,差异表达的lncRNA参与TGF-β信号通路和ABC转运体信号通路。
lncRNA主要通过表观遗传水平、转录及转录后水平调控基因的表达,在多种生理学和病理学过程中发挥重要作用[3]。对结核进行的相关研究已证实:lncRNA参与了MTB感染宿主的基因调控,并对细胞自噬及相关信号通路具有调控作用[13]。ABC转运蛋白是膜转运蛋白家族中的一类特殊蛋白质,介导多种底物分子在细胞内外的运输,与肺脏疾病在内的多种疾病的发生有关[14]。ABC转运蛋白家族成员中的ABCA1、ABCA5及ABCG1与细胞胆固醇转运有关。细菌内毒素脂多糖(LPS)能够下调巨噬细胞胆固醇转运蛋白ABCA1和ABCG1表达,从而影响细胞胆固醇的流出[15-17],对TLR信号介导的炎性反应发挥调控作用[18]。TGF-β信号通路可通过调节细胞的生长、增殖、分化、迁移和凋亡等过程,在机体的免疫反应中发挥重要作用。在结核病中,TGF-β能够抑制T细胞的反应,也能使巨噬细胞失活,是结核病免疫发病中的一个关键因素[19]。研究[20]表明:在结核肉芽肿中,TGF-β1是主要的细胞毒T细胞的抑制分子,而缺乏TGF-β1可增强T细胞对结核菌的清除作用。
在MTB感染后,宿主细胞主要通过模式识别受体(pattern recognition receptors,PRRs)来识别MTB,PRRs通过调节复杂的调控因子网络主导针对某一特异配体产生适宜的免疫反应,诱导激活以病原体清除或组织动态平衡修复为最终目的的炎症反应。在本研究中,GO富集结果中最显著富集项为参与免疫反应的GO:0006955,且IL-6、IL-7和IL-23A等促炎因子表达上调,表明在BCG感染后,可引发MTB炎性反应,发挥抗感染的作用。
| [1] | AN M, RYU D R, WON PARK J, et al. ULK1 prevents cardiac dysfunction in obesity through autophagy-meditated regulation of lipid metabolism[J]. Cardiovas Res, 2017, 113(10): 1137–1147. DOI:10.1093/cvr/cvx064 |
| [2] | WATERS WR, PALMER MV. Mycobacterium bovis infection of cattle and white-tailed deer:Translational research of relevance to human tuberculosis[J]. Ilar J, 2015, 56(1): 26–43. DOI:10.1093/ilar/ilv001 |
| [3] | OUYANG J, HU J, CHEN JL. lncRNAs regulate the innate immune response to viral infection[J]. Wiley Interdiscip Rev RNA, 2016, 7(1): 129–143. DOI:10.1002/wrna.1321 |
| [4] | FU Y, XU X, XUE J, et al. Deregulated lncRNAs in B cells from patients with active tuberculosis[J]. PLoS ONE, 2017, 12(1): 1–14. |
| [5] | YI Z, LI J, GAO K, et al. Identifcation of differentially expressed long non-coding RNAs in CD4+ T cells response to latent tuberculosis infection[J]. J Infect, 2014, 69(6): 558–568. DOI:10.1016/j.jinf.2014.06.016 |
| [6] | YANG X, YANG J, WANG J, et al. Microarray analysis of long noncoding RNA and mRNA expression profiles in human macrophages infected with ycobacterium tuberculosis[J]. Sci Rep, 2016, 14(6): 38963. |
| [7] | MASTERS S L, MIELKE L A, CORNISH A L, et al. Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production[J]. EMBO Rep, 2010, 11(8): 640–646. DOI:10.1038/embor.2010.93 |
| [8] | MAGEE D A, CONLON K M, NALPAS N C, et al. Innate cytokine profiling of bovine alveolar macrophages reveals commonalities and divergence in the response to Mycobacterium bovis and Mycobacterium tuberculosis infection[J]. Tuberculosis, 2014, 94(4): 441–450. DOI:10.1016/j.tube.2014.04.004 |
| [9] | HMAMA Z, PENA-DIZA S, JOSEPH S, et al. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis[J]. Immunol Rev, 2015, 264(1): 220–232. DOI:10.1111/imr.2015.264.issue-1 |
| [10] | MORACO A H, KORNFELD H. Cell death and autophagy in tuberculosis[J]. Semin Immunol, 2014, 26(6): 497–511. DOI:10.1016/j.smim.2014.10.001 |
| [11] | VAN DER WEL N, DAVA D, HOUBEN D, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells[J]. Cell, 2007, 129(7): 1287–1298. DOI:10.1016/j.cell.2007.05.059 |
| [12] | BACH H, PAPAVINASASUNDARAM K G, WONG D, et al. Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B[J]. Cell Host Microbe, 2008, 3(5): 316–322. DOI:10.1016/j.chom.2008.03.008 |
| [13] | PAWAR K, HANISCH C, PALMA VERA S E, et al. Down regulated lncRNA MEG3 eliminates mycobacteria in macrophages via autophagy[J]. Sci Rep, 2016, 14(6): 19416. |
| [14] | VAN DER DEEN M, DE VRIES E G, TIMENS W, et al. ATP-binding cassette (ABC) transporters in normal and pathological lung[J]. Respir Res, 2005. DOI:10.1186/1465-9921-6-59 |
| [15] | YIN K, LIAO D F, TANG C K. ATP-binding membrane cassette transporter A1(ABCA1):A possible link between inflammation and reverse cholesterol transport[J]. Mol Med, 2010, 16(9/10): 438–449. |
| [16] | CASTRILLO A, JOSEPH S B, VAIDYA S A, et al. Crosstalk between LXR and Toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism[J]. Mol Cell, 2003, 12(4): 805–816. DOI:10.1016/S1097-2765(03)00384-8 |
| [17] | KHOVIDHUNKIT W, KIM M S, MEMON R A, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism:mechanisms and consequences to the host[J]. Lipid Res, 2004, 45(7): 1169–1196. DOI:10.1194/jlr.R300019-JLR200 |
| [18] | AZZAM K M, FESSLER M B. Crosstalk between reverse cholesterol transport and innate immunity[J]. Trends Endocrin Met, 2012, 23(4): 169–178. DOI:10.1016/j.tem.2012.02.001 |
| [19] | 刘萍, 张丽, 庞楠楠, 等. 血清IFN-γ、TGF-β1、IL-10检测对儿童潜伏性结核感染的诊断价值[J]. 中国病原生物学杂志, 2011, 6(9): 650–652. |
| [20] | WARSINSKE H C, PIENAAR E, LINDERMAN J J, et al. Deletion of TGF-beta1 increases bacterial clearance by cytotoxic T cells in a tuberculosis granuloma model[J]. Front Immunol, 2017, 8: 1843. DOI:10.3389/fimmu.2017.01843 |
 2019, Vol. 45
2019, Vol. 45


