扩展功能
文章信息
- 于洋, 张岳培, 王润泽, 刘师兵, 徐路, 徐冶
- YU Yang, ZHANG Yuepei, WANG Runze, LIU Shibing, XU Lu, XU Ye
- 氯化钴对人卵巢癌SKOV3细胞顺铂敏感性的影响
- Influence of CoCl2 in cisplatin sensitivity of human ovarian cancer SKOV3 cells
- 吉林大学学报(医学版), 2019, 45(01): 1-6
- Journal of Jilin University (Medicine Edition), 2019, 45(01): 1-6
- 10.13481/j.1671-587x.20190101
-
文章历史
- 收稿日期: 2018-06-08
目前卵巢癌是女性生殖系统肿瘤致死的首要病因[1],主要是因其具有较高发病率的同时,临床化疗药物使用累积到一定剂量或使用一段时间后,出现肿瘤细胞对化疗药物敏感性的下降[2],因此对于肿瘤细胞敏感性下降具体原因的相关基础研究正逐渐成为肿瘤治疗学研究的焦点。肿瘤是由大量肿瘤细胞堆叠形成的实体组织,瘤体一般情况下是由大量的肿瘤细胞、血管、淋巴管和炎症细胞等形成的区别于人体正常组织环境的特殊微环境,其最大的特点之一就是长期处于低氧状态[3]。正常氧含量条件下细胞内低氧诱导因子(hypoxia inducible factor,HIF)的亚基之一HIF-1α也有表达,但是半衰期不足10 min[4],而在肿瘤细胞内,HIF-1α持续高表达较为普遍,其对肿瘤的增殖和迁移等都具有重要的调控作用[5-6],在低氧条件下能够激活多种靶基因如诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)、血管内皮生长因子(vascular endothelial growth factor,VEGF)和红细胞生成素(erythropoietin,EPO)等的转录,使细胞适应低氧环境[7-8],调节细胞生长[9]、转录和能量代谢[10],维持细胞氧平衡[11]。故以HIF-1α为靶点治疗肿瘤无疑是一个合理的思路。氯化钴(CoCl2)中的二价钴离子可通过阻止HIF-1α被羟基化而稳定细胞内HIF-1α的表达,因此常被用作建立细胞缺氧模型[12-14]。本研究将CoCl2作用于人卵巢癌SKOV3细胞株,特异性升高HIF-1α表达水平,首次将其下游iNOS表达和线粒体钙离子(Ca2+)变化作为观察目标,着重探讨其对线粒体凋亡的影响,以期明确CoCl2降低SKOV3细胞株顺铂(DDP)敏感性的相关机制,为临床肿瘤化疗提供理论依据。
1 材料与方法 1.1 细胞株、主要试剂和仪器人源性卵巢癌细胞株SKOV3由吉林大学病理生理学系惠赠。HIF-1α、iNOS、细胞色素C(cytochrome C,cyto C)、半胱氨酸天冬氨酸蛋白酶3(cysteinyl aspartase3,caspase3)和活化半胱氨酸天冬氨酸蛋白酶3(cleaved cysteinyl aspartase3,cleaved caspase3)一抗和二抗均购自美国Santa Cruz公司,线粒体Ca2+荧光探针Rhod 2-AM购自上海浩然生物技术有限公司,Muse®细胞状态分析仪凋亡检测试剂盒购于美国Muse®试剂公司,其他化学试剂购于北京鼎国生物试剂公司。860型酶标仪(美国Bio-Rad公司),化学发光仪(中国上海天能科技有限公司),FV1000型共聚焦显微镜(日本Olympus公司),Muse®细胞状态分析仪(美国默克密理博公司)。
1.2 细胞培养和分组SKOV3细胞用含体积分数为10%新生牛血清的1640培养液,置37℃、5% CO2培养箱中培养,隔2~3 d传代1次,取生长状态良好处于对数生长期细胞随机分为对照组、CoCl2组、DDP组和联合(CoCl2联用DDP)组。对照组细胞不做处理,CoCl2组细胞以200 μmol·L-1CoCl2作用4 h后换成常规培养基继续培养20 h,DDP组细胞以含10 mg·L-1 DDP的培养基培养24 h,联合组细胞以200 μmol·L-1CoCl2作用4 h后更换含10 mg·L-1DDP的细胞培养液继续培养20 h。
1.3 MTT法检测各组细胞存活率细胞接种至96孔板,按1.2中的方法进行分组及给药处理24 h后,每孔加入10 μL浓度为5 g·L-1MTT溶液,继续培养4 h。弃去上清,每孔加入100 μL二甲基亚砜,置摇床上低速振荡10 min,使结晶物充分溶解。最后以酶标仪在490 nm处检测各孔吸光度(A)值,计算细胞存活率。细胞存活率=(给药组A值-空白组A值)/(对照组A值-空白组A值)× 100%。
1.4 间接免疫荧光法检测各组细胞HIF-1α和iNOS阳性表达强度按1.2中的方法进行分组及给药处理,制备细胞爬片,4%多聚甲醛固定30 min,0.01 mol·L-1PBS洗3次,每次2 min。加入蛋白酶K 1 mg·L-1,消化1 min,0.01 mol·L-1 PBS洗3次,每次2 min;0.1% Triton孵育细胞10 min,0.01 mol·L-1PBS洗3次,每次2 min;5%山羊血清封闭30 min后,弃去多余血清,将稀释好的HIF-1α和iNOS一抗覆在细胞面上,4℃湿盒过夜。次日,弃去多余一抗,0.01%Triton-0.01 mol·L-1PBS洗3次,每次5 min,以相应来源的荧光标记二抗室温避光孵育40 min,0.01% Triton-0.01 mol·L-1 PBS洗3次,每次5 min,Hoechst33342复染细胞核2 min,甘油封片。激光共聚焦显微镜观察HIF-1α(绿色光)和iNOS(红色光)阳性表达强度。每张细胞爬片随机选取5个视野,以阳性表达荧光亮度值代表HIF-1α和iNOS阳性表达强度。
1.5 线粒体Ca2+水平测定按1.2中的方法进行分组及给药处理,制备细胞爬片,以5 μmol·L-1荧光钙探针Rhod 2-AM 37℃培养箱孵育30 min,0.01 mol·L-1 PBS洗3次,每次2 min,甘油封片,共聚焦显微镜下观察。红色荧光显示线粒体内的Ca2+分布,荧光亮度越强说明线粒体Ca2+水平越高。每张细胞爬片随机选取5个视野,检测红色荧光亮度值代表线粒体Ca2+水平。
1.6 Western blotting法检测各组细胞中cyto C、caspase3和cleaved caspase3蛋白表达水平按1.2中的方法分组处理SKOV3细胞,以胰蛋白酶消化、离心,收集全部细胞,收集后的每管细胞加入120 μL RIPA细胞裂解液,混匀后,冰浴下用细胞超声粉碎仪进行超声粉碎2次,每次5~10 s,然后放入4℃冰箱45 min,充分裂解细胞。离心,收集上清液,以96孔板蛋白定量后,所余部分上清液与5×SDS Loading缓冲液按4︰1体积比混匀后进行蛋白变性。变性后的蛋白提取液进行SDS-PAGE电泳,电泳条件:浓缩胶100V、30 min,分离胶200V、60 min,电泳结束后,利用湿转法将胶内蛋白转移到PVDF膜上,将膜用5%脱脂奶粉封闭1.5 h后,PBST洗3次,加入以一抗稀释液200倍稀释后的检测目标一抗(分别为β-actin、cyto C、caspase3和cleaved caspase3),4℃孵育过夜。次日,PBST洗3次,加入过氧化物酶标记的相应二抗(1︰1 000稀释),室温下摇床摇1 h,PBST洗3次,ECL发光并拍照,以β-actin作为内参照对各组蛋白灰度值进行对比分析,每组蛋白重复3次。目的蛋白表达水平=每个样本条带灰度值/β-actin灰度值。
1.7 Muse凋亡试剂盒检测各组细胞凋亡率采用Muse®细胞状态分析仪,严格按照凋亡检测试剂盒说明书进行操作。每组样品做5个平行样,测得凋亡率数据进行统计学分析。细胞凋亡率=早期凋亡率+晚期凋亡率。
1.8 统计学分析统计图表用Excel软件绘制。采用SPSS 13.0统计软件进行统计学分析。各组SKOV3细胞存活率,细胞中HIF-1α和iNOS阳性表达强度,细胞中Ca2+水平,细胞中cyto C、caspase3、cleaved caspase3蛋白表达水平和细胞凋亡率,均以x±s表示,多组间样本均数比较采用单因素方差分析,均数间多重比较采用LSD-t检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组SKOV3细胞存活率与对照组(100.00%±1.24%)比较,CoCl2组细胞存活率无明显变化(P > 0.05),DDP组细胞存活率(53.64%±9.23%)和联合组细胞存活率(72.17%±8.28%)明显下降(t=-9.36,P < 0.05;t=-10.15,P < 0.05),且联合组高于DDP组(t=6.68,P < 0.05)。
2.2 各组SKOV3细胞中HIF-1α和iNOS阳性表达强度间接免疫荧光法观察各组细胞HIF-1α和iNOS表达情况见图 1(插页一)。对照组和DDP组细胞中有少量HIF-1α荧光阳性表达(绿色荧光处)。与对照组比较,CoCl2组和联合组细胞中HIF-1α阳性表达强度明显增强(t=7.15,P < 0.05;t=8.32,P < 0.05),CoCl2组细胞中iNOS阳性表达强度(红色荧光处)无明显变化(P > 0.05),DDP组和联合组细胞中iNOS阳性表达强度明显增强(t=10.21,P < 0.05;t=12.58,P < 0.05)。与CoCl2组比较,DDP组和联合组细胞中HIF-1α阳性表达强度明显降低(t=-4.35,P < 0.05;t=-6.28,P < 0.05),iNOS阳性表达强度明显增强(t=9.21,P < 0.05;t=11.43,P < 0.05)。与DDP组比较,联合组细胞中HIF-1α阳性表达强度明显增强(t=4.76,P < 0.05),iNOS阳性表达强度明显降低(t=-8.24,P < 0.05)。见表 1。
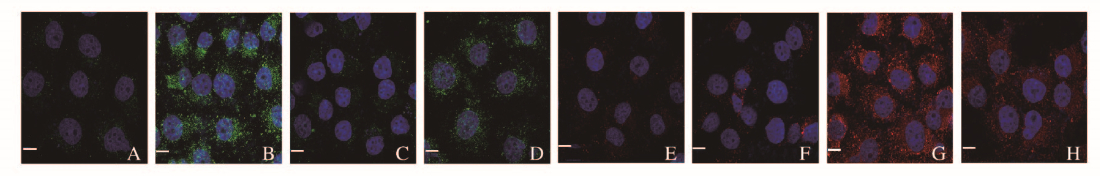
|
| A-D: HIF-1α; E-H: iNOS; A, E: Control group; B, F: CoCl2 group; C, G: DDP group; D, H: Combination group. 图 1 间接免疫荧光法检测各组SKOV3细胞中HIF-1α和iNOS阳性表达(Bar=10 μm) Fig. 1 Expressions of HIF-1α and iNOS in SKOV3 cells in various groups detected by indirect immunofluorescence method (Bar=10 μm) |
|
|
| (n=5, x±s) | ||
| Group | HIF-1α | iNOS |
| Control | 1.47±0.31 | 3.15±0.12 |
| CoCl2 | 53.28±2.16* | 4.69±0.66 |
| DDP | 3.94±0.82△ | 62.58±4.76*△ |
| Combination | 12.76±2.12*△# | 21.22±5.69*△# |
| *P < 0.05 vs control group; △P < 0.05 vs CoCl2 group; #P < 0.05 vs DDP group. | ||
利用Rhod 2-AM荧光探针观察各组细胞线粒体Ca2+变化(图 2,见插页一),红色荧光亮度代表线粒体中Ca2+水平。与对照组(2.17±0.26)比较,CoCl2组细胞线粒体中Ca2+水平(3.28±0.52)无明显变化(P > 0.05),DDP组和联合组细胞线粒体中Ca2+水平(72.56±9.68和25.47±7.85)明显升高(t=14.26,P < 0.05);与DDP组比较,CoCl2组和联合组细胞线粒体中Ca2+水平明显降低(t=-11.47,P < 0.05;t=-9.68,P < 0.05)。

|
| A: Control group; B: CoCl2 group; C: DDP group; D: Combination group. 图 2 Rhod 2-AM荧光探针检测各组SKOV3细胞线粒体Ca2+水平(Bar=100 μm) Fig. 2 Levels of mitochondrial Ca2+ in SKOV3 cells in various groups detected by Rhod 2-AM fluorescent probe (Bar=100 μm) |
|
|
与对照组比较,CoCl2组细胞中cyto C、caspase3和cleaved caspase3表达水平无明显变化(P > 0.05),DDP组细胞中cyto C、caspase3和cleaved caspase3表达水平明显升高(t=3.14,P < 0.05;t=4.28,P < 0.05;t=9.65,P < 0.05);与DDP组比较,联合组cyto C(t=-4.32,P < 0.05)、caspase3(t=-8.74,P < 0.05)和cleaved caspase3(t=-10.23,P < 0.05)表达水平明显降低。见图 3。
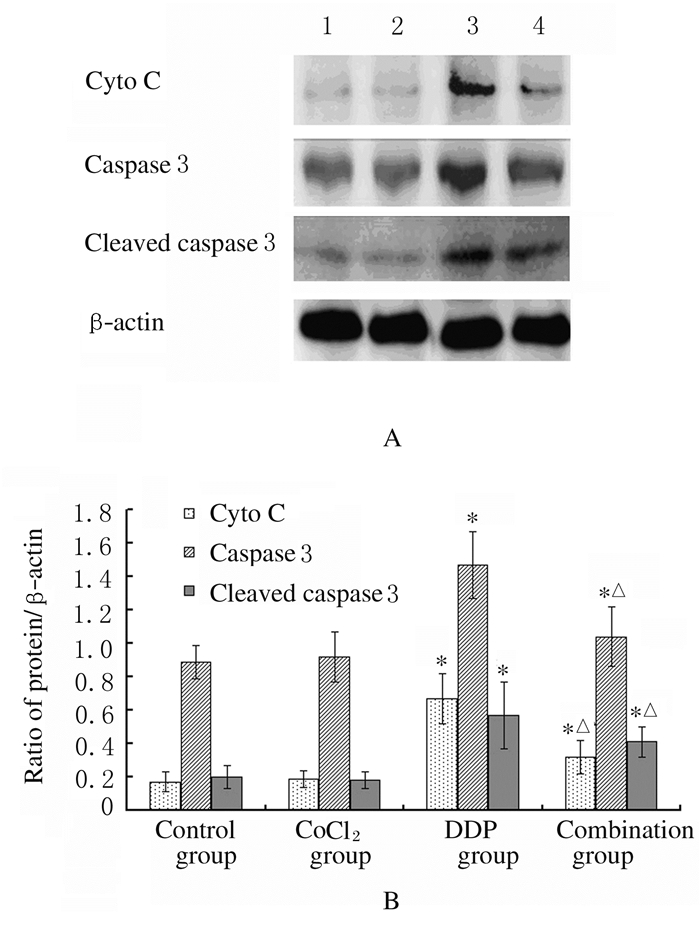
|
| Lane 1:Control group; Lane 2:CoCl2 group; Lane 3:DDP group; Lane 4:Combined group. *P < 0.05 vs control group; △P < 0.05 vs DDP group. 图 3 各组SKOV3细胞中cyto C、caspase3和cleaved caspase3蛋白表达电泳图(A)和直条图(B) Fig. 3 Electrophorogram (A) and histogram (B) of expressions of cyto C, caspase3 and cleaved caspase3 proteins in SKOV3 cells in various groups |
|
|
与对照组细胞凋亡率(2.89%±1.12%)比较,CoCl2组细胞凋亡率(4.37%±1.48%)无明显变化(P > 0.05),DDP组和联合组细胞凋亡率(49.17%±9.36%和25.21%±4.94%)明显升高(t=8.57,P < 0.05;t=7.89,P < 0.05);与DDP组比较,联合组细胞凋亡率明显降低(t=-9.33,P < 0.05)。见图 4。
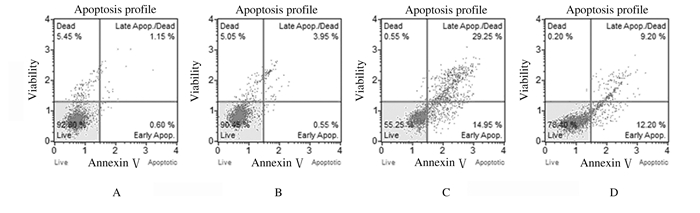
|
| A: Control group; B: CoCl2 group; C: DDP group; D: Combination group. 图 4 Muse®凋亡试剂盒检测各组SKOV3细胞凋亡率 Fig. 4 Apoptosis rates of SKOV3 cells in various groups detected by Muse® apoptosis assay kit |
|
|
WANG等[15]于1993年首次发现HIF-1的存在,是由α和β2个亚单位形成的二聚体,之后关于其相关作用和功能的研究便逐渐展开。研究[7, 16-17]显示:HIF-1α表达增强可影响iNOS表达,但iNOS表达水平升高的意义和机制尚未明确。在诸多相关细胞或组织缺血、缺氧的研究[18-19]中均显示:iNOS作为重要的信号分子参与组织及细胞的氧化水平调节和氧化损伤代偿,但对于iNOS表达的调控机制尚不十分完善。本课题组前期研究[20]显示:iNOS能够通过调节SKOV3和SKOV3/DDP细胞株线粒体Ca2+水平对其DDP耐药性造成影响,推测HIF-1α可能通过影响iNOS表达来影响SKOV3细胞株的DDP耐药性。本研究结果显示:CoCl2能够诱导SKOV3细胞株HIF-1α表达增强,但对正常情况下SKOV3细胞中iNOS表达强度影响甚微,而在DDP诱导iNOS表达强度明显增高时,CoCl2对iNOS表达增强呈现出明显的抑制作用,即CoCl2能够降低DDP对于SKOV3细胞iNOS的诱导作用,同时本研究中关于线粒体中Ca2+水平的变化也就得到了相应的解释。钙作为传递细胞信号重要信使,通常储存在内质网,感应到细胞信号时可以由内质网流入线粒体,启动线粒体通路细胞凋亡程序[21]。本研究结果显示:SKOV3细胞线粒体Ca2+水平高低在与iNOS表达趋势一致的同时,也与细胞凋亡率和凋亡相关蛋白表达水平有关,说明DDP对SKOV3细胞的凋亡诱导作用可以通过诱导iNOS表达增强从而升高线粒体Ca2+水平,启动线粒体途径凋亡得以实现;而当CoCl2参与其中使HIF-1α表达增强时,则抑制了DDP诱导的SKOV3细胞株iNOS表达增强,进而影响SKOV3细胞对DDP的敏感性。上述结论亦可以从各组SKOV3细胞存活率、凋亡率、线粒体凋亡相关蛋白cyto C、caspase 3和cleaved caspase 3表达水平的变化中得以验证。
综上所述,CoCl2可以升高HIF-1α表达水平,抑制DDP诱导的SKOV3细胞中iNOS表达增强,调节由于iNOS表达增强引发的线粒体Ca2+水平升高,减少线粒体途径凋亡的发生,从而降低人卵巢癌SKOV3细胞DDP敏感性。
| [1] | LALRINPUⅡ E, BHAGEERATHY P S, SEBASTIAN A, et al. Ovarian cancer in young women[J]. Indian J Surg Oncol, 2017, 8(4): 540–547. DOI:10.1007/s13193-017-0680-z |
| [2] | MUINAO T, DEKA BORUAH H P, PAL M. Diagnostic and prognostic biomarkers in ovarian cancer and the potential roles of cancer stem cells-An updated review[J]. Exp Cell Res, 2018, 362(1): 1–10. DOI:10.1016/j.yexcr.2017.10.018 |
| [3] | SHABANA A M, MONDAL U K, ALAM M R, et al. pH-Sensitive multiligand gold nanoplatform targeting carbonic anhydrase Ⅸ enhances the delivery of doxorubicin to hypoxic tumor spheroids and overcomes the hypoxia-Induced chemoresistance[J]. ACS Appl Mater Interfaces, 2018, 10(21): 17792–17808. DOI:10.1021/acsami.8b05607 |
| [4] | FU J, ZHANG J, GONG Y, et al. Regulation of HIF-1 alpha by the proprotein convertases furin and PC7 in human squamous carcinoma cells[J]. Mol Carcinog, 2015, 54(9): 698–706. DOI:10.1002/mc.22131 |
| [5] | COURTNAY R, NGO D C, MALIK N, et al. Cancer metabolism and the Warburg effect:the role of HIF-1 and PI3K[J]. Mol Biol Rep, 2015, 42(4): 841–851. DOI:10.1007/s11033-015-3858-x |
| [6] | SONG X, YANG C, ZHANG H, et al. Hypoxia-inducible factor-1α (HIF-1α) expression on endothelial cells in juvenile nasopharyngeal angiofibroma:A review of 70 cases and tissue microarray analysis[J]. Ann Otol Rhinol Laryngol, 2018, 127(6): 357–366. DOI:10.1177/0003489418765563 |
| [7] | CHENG Y, FENG Y, XIA Z, et al. ω-Alkynyl arachidonic acid promotes anti-inflammatory macrophage M2 polarization against acute myocardial infarction via regulating the cross-talk between PKM2, HIF-1α and iNOS[J]. Biochim Biophys Acta, 2017, 1862(12): 1595–1605. DOI:10.1016/j.bbalip.2017.09.009 |
| [8] | BARBEN M, SAMARDZIJA M, GRIMM C. The role of hypoxia, hypoxia-inducible factor (HIF), and VEGF in retinal angiomatous proliferation[J]. Adv Exp Med Biol, 2018, 1074: 177–183. DOI:10.1007/978-3-319-75402-4 |
| [9] | ZHANG X, LUO H. Effects of thalidomide on growth and VEGF-A expression in SW480 colon cancer cells[J]. Oncol Lett, 2018, 15(3): 3313–3320. |
| [10] | JIN P, KANG J, LEE M K, et al. Ferritin heavy chain controls the HIF-driven hypoxic response by activating the asparaginyl hydroxylase FIH[J]. Biochem Biophys Res Commun, 2018, 499(3): 475–481. DOI:10.1016/j.bbrc.2018.03.173 |
| [11] | CHEN P J, WENG J Y, HSU P H, et al. NPGPx modulates CPEB2-controlled HIF-1α RNA translation in response to oxidative stress[J]. Nucleic Acids Res, 2015, 43(19): 9393–3404. DOI:10.1093/nar/gkv1010 |
| [12] | CHEN R, XU J, SHE Y, et al. Necrostatin-1 protects C2C12 myotubes from CoCl2-induced hypoxia[J]. Int J Mol Med, 2018, 41(5): 2565–2572. |
| [13] | PECORARO M, PINTO A, POPOLO A. Inhibition of Connexin 43 translocation on mitochondria accelerates CoCl2-induced apoptotic response in a chemical model of hypoxia[J]. Toxicol In Vitro, 2018, 47(3): 120–128. |
| [14] | ZHANG M, MA R, LI Q. Inhibitory action of CoCl2-induced MCF-7 cell hypoxia model of breast cancer and its influence on vascular endothelial growth factor[J]. J Biol Regul Homeost Agents, 2015, 29(3): 671–676. |
| [15] | WANG G L, SEMENZA G L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia[J]. Proc Natl Acad Sci U S A, 1993, 90(9): 4304–4308. DOI:10.1073/pnas.90.9.4304 |
| [16] | HSU J T, LE P H, LIN C J, et al. Mechanism of salutary effects of melatonin-mediated liver protection after trauma-hemorrhage:p38 MAPK-dependent iNOS/HIF-1α pathway[J]. Am J Physiol Gastrointest Liver Physiol, 2017, 312(5): 427–433. DOI:10.1152/ajpgi.00440.2016 |
| [17] | PEÑA-MERCADO E, GARCIA-LORENZANA M, ARECHAGE-OCAMPO E, et al. Evaluation of HIF-1α and iNOS in ischemia/reperfusion gastric model:bioimpedance, histological and immunohistochemical analyses[J]. Histol Histopathol, 2018, 33(8): 815–823. |
| [18] | RIEMANN A, REIME S, THEWS O. Tumor acidosis and hypoxia differently modulate the inflammatory program:measurements in vitro and in vivo[J]. Neoplasia, 2017, 19(12): 1033–1042. DOI:10.1016/j.neo.2017.09.005 |
| [19] | CASILLAN A J, CHAO J, WOOD J G, et al. Acclimatization of the systemic microcirculation to alveolar hypoxia is mediated by an iNOS-dependent increase in nitric oxide availability[J]. J Appl Physiol, 2017, 123(4): 974–982. DOI:10.1152/japplphysiol.00322.2016 |
| [20] | YU Y, XIE Q, LIU W, et al. Increased intracellular Ca2+ decreases cisplatin resistance by regulating iNOS expression in human ovarian cancer cells[J]. Biomed Pharmacother, 2017, 86(2): 8–15. |
| [21] | BERTERO E, MAACK C. Calcium signaling and reactive oxygen species in mitochondria[J]. Circ Res, 2018, 122(10): 1460–1478. DOI:10.1161/CIRCRESAHA.118.310082 |
 2019, Vol. 45
2019, Vol. 45


