扩展功能
文章信息
- 王廷刚, 薛峰, 李宇, 牛兆健, 王野
- WANG Tinggang, XUE Feng, LI Yu, NIU Zhaojian, WANG Ye
- miR-26a靶向HMGA1基因对结肠癌细胞生长、侵袭和迁移的影响
- Effects of miR-26a targeting HMGA1 gene on growth, invasion and migration of colon cancer cells
- 吉林大学学报(医学版), 2018, 44(06): 1205-1211
- Journal of Jilin University (Medicine Edition), 2018, 44(06): 1205-1211
- 10.13481/j.1671-587x.20180617
-
文章历史
- 收稿日期: 2018-05-21
2. 青岛大学医学院附属医院普外科, 山东 青岛 266000
2. Department of General Surgery, Affiliated Hospital, College of Medical Sciences, Qiingdao University, Qingdao 266000, China
结肠癌是常见的发生于消化系统的恶性肿瘤,其发病率和死亡率分别位居恶性肿瘤第2位和第4位。目前对结肠癌的形成、发展、治疗和预后虽有新的认识,但仍未发现合适的标记物用于分析结肠癌的发病机制、诊断和治疗[1-2]。
结肠癌的发生是一个多步骤、多因素和多阶段的过程,目前对结肠癌的研究[3]主要集中在mRNA和蛋白水平。微小RNA(microRNA,miRNA)是一类长度为18~25个核苷酸的小分子非编码RNA,可在转录后调控基因的表达,目前关于miRNA抑制肿瘤发生的研究[4]逐渐增多,miR-26a是miRNA的一种,其在肿瘤中作用的研究[5]也明显增多。研究[6]显示:在鼻咽癌组织和细胞中miR-26a表达水平降低,并通过靶向EZH2基因抑制癌细胞的生长和致瘤性。高迁移率族蛋白1(high mobility group protein1,HMGA1)是miR-26a的一个靶基因,miR-26a可靶向HMGA1调控膀胱癌细胞的生长、凋亡、侵袭和迁移[7]。作为HMGA家族一员,HMGA1在人类各种肿瘤发生发展中起重要作用[8]。miR-26a在不同癌症中的功能与其靶标有关,关于其靶向调节HMGA1对结肠癌细胞的生物学特性的影响机制尚不清楚。因此,本研究旨在探讨miR-26a靶向HMGA1对结肠癌细胞生长、侵袭和迁移的影响,以期为后续研究提供依据。
1 材料与方法 1.1 细胞、主要试剂和仪器人结肠癌SW480细胞购自中国科学院细胞库。胎牛血清、RPMI1640培养基、胰酶和青链霉素购自美国Gibco公司,BCA和CCK8试剂盒购自中国碧云天生物技术有限公司,SYBR Green购自上海生工生物工程有限公司,双荧光报告基因检测试剂盒购自美国Promega公司,miR-26a mimics、miR-26ainhibitor和miR-NC购自广州锐博生物科技有限公司,pcDNA3.1载体购自美国Invitrogen公司,PGL3荧光素酶报告基因载体购自北京普洛麦格生物技术有限公司。CO2细胞培养箱购自美国Thermo公司,倒置荧光显微镜购自日本Olympus公司,实时定量PCR仪购自美国ABI公司。
1.2 细胞培养将装有人结肠癌SW480细胞的冻存管从液氮罐中取出,37℃水浴解冻后,采用移液枪将细胞转移到5 mL无菌离心管中,加入含有10%FBS、100 mg·L-1链霉素和100 U·L-1青霉素的RPMI1640培养基中,1 000 r·min-1离心5 min,弃去上清,加入细胞培养液悬浮细胞,接种到细胞培养板中,置于37℃、5% CO2培养箱中培养。细胞贴壁后换液1次,之后每2~3 d换液1次,细胞融合度达到80%~90%时进行传代。
1.3 细胞转染和细胞中HMGA1mRNA和蛋白表达水平检测取生长至对数期的人结肠癌SW480细胞,加入0.25 %胰酶消化细胞,消化完全后将细胞转移到新的离心管中,1 000 r·min-1离心5 min,弃去上清,加入不含有胎牛血清的不完全培养液接种到细胞培养板中,在37℃、5% CO2培养箱中培养。当细胞融合度达60%时,将miR-NC(miR-NC组)、miR-26a mimics(模拟物,miR-26amimics组)和miR-26a inhibitor(抑制物,miR-26a inhibitor组)与不完全培养基混合后加入至人结肠癌SW480细胞中,滴加至24孔板表面,37℃、5% CO2培养箱中培养48 h。采用定量RT-PCR(qRT-PCR)和Western blotting法检测SW480细胞中HMGA1mRNA和蛋白表达水平。
1.4 荧光素酶报告基因检测各组SW480细胞中双荧光素酶活性通过TargetScan( http://www.targetscan.org/)、PicTa(http://pictar.mdc-berlin.de/)和The miRBase(http://mirbase.org/)三大靶基因预测库预测到miR-26a与HMGA1的3′-UTR有结合位点。将含有miR-26a结合位点HMGA1的3′-UTR片段插入PGL3荧光素酶报告基因载体,构建野生型和突变型HMGA1的3′-UTR荧光素酶报告载体,分别命名为Wt-HMGA1和Mut-HMGA1。将构建好的质粒进行共转染并分为3组,即miR-NC+miR-26a mimics组、Wt-HMGA1+miR-26a mimics组和Mut-HMGA1+miR-26a mimics组。置于37℃、5%CO2培养箱中培养24 h后收集细胞,采用双荧光素酶报告基因检测试剂盒检测各组SW480细胞中双荧光素酶活性。
1.5 CCK8法检测人结肠癌SW480细胞增殖活力取转染后生长至对数期的miR-NC组、miR-26a mimics组和miR-26a inhibitor组细胞,采用细胞培养液悬浮细胞,细胞浓度调整为3×105mL-1,接种于96孔细胞培养板中,每孔加入100 μL细胞悬液,置于37℃、5% CO2培养箱中培养24、48和72 h后,加入10 μL CCK8溶液,置于37℃、5% CO2培养箱孵育2 h,酶标仪在490 nm波长处测定并记录各组吸光度(A)值,以A值反映细胞的增殖活力。
1.6 CCK8法检测转染后细胞增殖活力将人结肠癌SW480细胞分为miR-NC组(未转染)、miR-26a mimics组(未转染)、miR-26a mimics+pcDNA3.1-HMGA1质粒共转染组(miR-26a mimics转染pcDNA3.1-HMGA1质粒)和miR-NC+pcDNA3.1-HMGA1质粒共转染组(miR-NC转染pcDNA3.1-HMGA1质粒)。取各组对数生长期的人结肠癌SW480细胞,采用0.25 %胰酶消化细胞,转移细胞至新的EP管中,1 000 r·min-1离心5 min,弃上清,与不完全培养液接种到细胞培养板中,置于37℃、5% CO2培养箱中培养24、48和72 h后,参照1.3步骤检测各组细胞的A值,以A(490)/A(630)反映各组细胞的增殖活力。
1.7 Transwell小室法检测各组SW480细胞中侵袭细胞数将细胞分为miR-NC组、miR-26a mimics组、miR-26a mimics+pcDNA3.1-HMGA1质粒共转染组和miR-NC+pcDNA3.1-HMGA1质粒共转染组。取生长至对数期的人结肠癌SW480细胞,调整细胞浓度为1×105mL-1,取100 μL细胞悬液接种于覆盖有Matrigel基质胶的Transwell小室的上室,下层加入含10% FBS的RPMI1640培养基500 μL,培养72 h后弃去培养液,用棉签轻轻拭掉膜上的细胞,HE染色后在倒置显微镜下观察各组侵袭细胞数。
1.8 Transwell小室法检测各组SW480细胞中迁移细胞数将细胞分为miR-NC组、miR-26a mimics组、miR-26a mimics+pcDNA3.1-HMGA1质粒共转染组和miR-NC+pcDNA3.1-HMGA1质粒共转染组。除了Transwell室上未铺Matrigel基质胶外,其他的操作如同1.7步骤。
1.9 统计学分析采用SPSS20.0统计软件进行统计学分析。各组人结肠癌SW480细胞中HMGA1 mRNA表达水平、细胞增殖活力、侵袭细胞数和迁移细胞数以x±s表示,组间比较采用t检验,组内两两比较采用SNK-q检验。检验水准α=0.05。
2 结果 2.1 各组SW480细胞中HMGA1 mRNA和蛋白表达水平及荧光素酶活性miR-26a mimics组SW480细胞中HMGA1 mRNA表达水平(0.273± 0.092)低于miR-NC组(0.992±0.112)(t=8.592,P=0.001),miR-26a inhibitor组SW480细胞中HMGA1 mRNA表达水平(3.754±0.241)高于miR-NC组(t=18.001,P=0.000),Western blotting检测各组SW480细胞中HMGA1蛋白表达水平见图 1~3。Wt- HMGA1+miR-26a mimics共转染组荧光素酶活性(0.383±0.054)低于miR-NC+miR-26a mimics共转染组(1.030±0.071)(t=12.563,P=0.000),而Mut-HMGA1+miR-26a mimics共转染组与miR-NC+miR-26a mimics共转染组(1.001±0.081)SW480细胞中荧光素酶活性比较差异无统计学意义(t=0.466,P=0.665),见图 4,即HMGA1是miR-26a的靶基因。
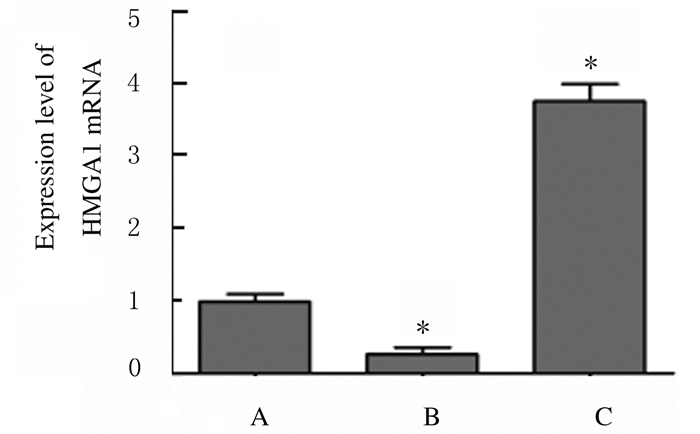
|
| A:miR-NC group; B:miR-26a mimics group; C:miR-26a inhibitor group.*P<0.05 vs miR-NC group. 图 1 各组SW480细胞中HMGA1 mRNA表达水平 Figure 1 Expression levels of HMGA1 mRNA in SW480 cells in various groups |
|
|

|
| Lane 1:miR-NC+miR-26a inhibitor group; Lane 2:Wt-HMGA1+miR-26a mimics group; Lane 3:Mut-HMGA1+miR-26a mimics group. 图 2 各组SW480细胞中HMGA1蛋白表达电泳图 Figure 2 Electrophoregram of HMGA1 protein in SW480 cells in various groups |
|
|
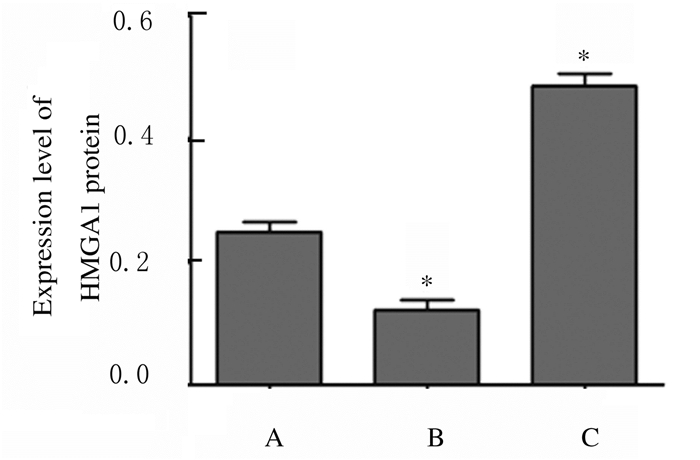
|
| A:miR-NC group; B:miR-26a mimics group; C:miR-26a inhibitor group.*P < 0.01 vs miR-NC group. 图 3 各组SW480细胞中HMGA1蛋白表达水平 Figure 3 Expression levels of HMGA1 protein in SW480 cells in various groups |
|
|
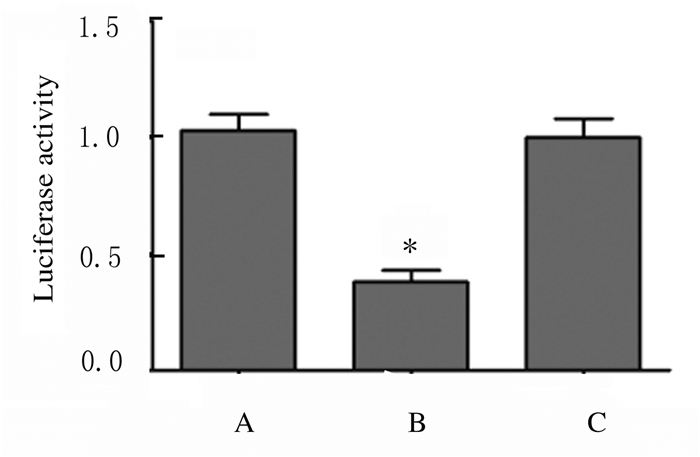
|
| A:miR-NC+miR-125a mimics; B:Wt-HMGA1+miR-125a mimics; C:Mutt-HMGA1+miR-125a mimics.*P < 0.01 vs miR-NC+miR-26a mimics group. 图 4 各组SW480细胞中双荧光素酶活性 Figure 4 Duble luciferase activities of SW480 cells in various groups |
|
|
miR-NC、miR-26a mimics和miR-26a inhibitor组细胞转染24(F=15.516,P=0.004)、48(F=41.849,P=0.000)和72 h(F=85.050,P=0.000)后细胞增殖活力比较差异均有统计学意义,miR-26a mimics组细胞增殖活力在上述3个时间点均明显低于miR-NC组(t24=2.566,P24=0.043;t48=4.721,P48=0.003;t72=6.547,P72=0.001),而miR-26a inhibitor组细胞增殖活力在上述3个时间点均明显高于miR-NC组(t24=2.999,P24=0.024;t48=4.426,P48=0.004;t72=6.496,P72=0.001)。见图 5和表 1。
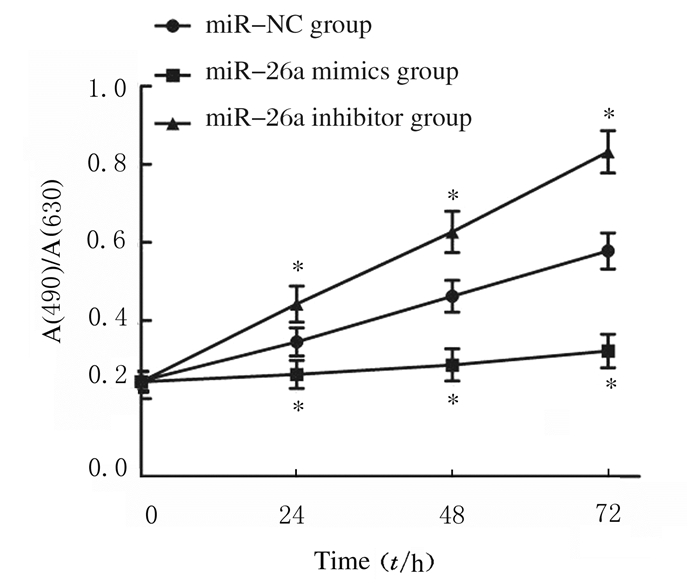
|
| *P < 0.05 vs miR-NC group. 图 5 CCK8法检测各组细胞增殖活力 Figure 5 Proliferation activities of cells in various groups detected by CCK8 method |
|
|
| (n=3, x±s) | |||
| Group | Prolieration activity | ||
| (t/h) 24 | 48 | 72 | |
| miR-NC | 0.345±0.036 | 0.462±0.041 | 0.578±0.046 |
| miR-26a mimics | 0.262±0.036* | 0.286±0.042* | 0.322±0.043* |
| miR-26a inhibitor | 0.442±0.046* | 0.627±0.053* | 0.832±0.054* |
| F | 15.516 | 41.849 | 85.050 |
| P | 0.004 | 0.000 | 0.000 |
| * P < 0.05 vs miR-NC group. | |||
miR-NC组、miR-26a mimics组、miR-26a mimics+pcDNA3.1-HMGA1组和miR-NC+pcDNA3.1-HMGA1组细胞转染24(F=56.233,P=0.000)、48(F=60.131,P=0.000)和72 h(F=78.398,P=0.000)后SW480细胞增殖活力比较差异均有统计学意义;miR-26a mimics组(t24=7.537,P24=0.000;t48=8.265,P48=0.000;t72=10.416,P72=0.000)、miR-26a mimics+pcDNA3.1-HMGA1组(t24=2.895,P24=0.028;t48=4.216,P48=0.003;t72=5.149,P72=0.001)细胞增殖活力在3个时间点均明显低于miR-NC组,miR-NC+pcDNA3.1-HMGA1组细胞增殖活力在3个时间点均明显高于miR-NC组(t24=4.264,P24=0.003;t48=4.484,P48=0.002;t72=4.002,P72=0.004);miR-26a mimics+pcDNA3.1-HMGA1组细胞增殖活力在3个时间点明显高于miR-26a mimics组(t24=4.427,P24=0.002;t48=4.049,P48=0.004;t72=5.267,P72=0.001),低于miR-NC+pcDNA3.1-HMGA1组(t24=7.026,P24=0.000;t48=8.700,P48=0.002;t72=9.151,P72=0.004)。见表 2。
| (n=3, x±s) | |||
| Group | Proliferation activity | ||
| (t/h) 24 | 48 | 72 | |
| miR-NC | 0.366±0.036 | 0.538±0.041 | 0.697±0.046 |
| miR-26a mimics | 0.206±0.036*△ | 0.291±0.041*△ | 0.343±0.043*△ |
| miR-26a mimics +pcDNA3.1-HMGA1 | 0.469±0.031* | 0.412±0.034* | 0.522±0.039* |
| miR-NC + pcDNA3.1-HMGA1 | 0.625±0.034*△ | 0.672±0.029*△ | 0.833±0.038*△ |
| F | 79.073 | 60.131 | 78.398 |
| P | 0.000 | 0.000 | 0.000 |
| * P < 0.01 vs miR-NC group; △ P < 0.01 vs miR-26a mimics + pcDNA3.1-HMGA1 group. | |||
miR-NC组、miR-26a mimics组、miR-26a mimics+ pcDNA3.1-HMGA1组和miR-NC+pcDNA3.1-HMGA1组细胞转染72 h后,Transwell小室法检测细胞侵袭结果显示:miR-26a mimics组和miR-26a mimics+pcDNA3.1-HMGA1组侵袭细胞数均明显低于miR-NC组(t1=12.953,P1=0.000;t2=4.741,P2=0.002),miR-NC+pcDNA3.1-HMGA1组侵袭细胞数明显高于miR-NC组(t=4.956,P=0.001);miR-26a mimics+pcDNA3.1-HMGA1组侵袭细胞数明显高于miR-26a mimics组(t=8.212,P=0.000),低于miR-NC+pcDNA3.1-HMGA1组(t=9.697,P=0.000)。见表 3。
| Group | Number of invasion cells | Number ofmigration cells |
| miR-NC | 139.8±5.6 | 189.8±8.7 |
| miR-26a mimics | 67.4±6.2* | 97.4±9.6* |
| miR-26a mimics+ pcDNA3.1-HMGA1 | 113.3±7.1*△# | 147.3±10.5*△# |
| miR-NC+ pcDNA3.1-HMGA1 | 167.5±8.2* | 239.2±13.8* |
| F | 116.168 | 93.540 |
| P | 0.000 | 0.000 |
| * P < 0.01 vs miR-NC group; △ P < 0.01 vs miR-26a mimics group; # P < 0.01 vs miR-NC+pcDNA3.1-HMGA1 group. | ||
miR-NC组、miR-26a mimics组、miR-26a mimics+ pcDNA3.1-HMGA1组和miR-NC+pcDNA3.1-HMGA1组细胞转染72 h后,Transwell小室法检测结果显示:miR-26a mimics组和miR-26a mimics+pcDNA3.1-HMGA1组迁移细胞数均明显低于miR-NC组(t1=10.456,P1=0.000;t2=4.809,P2=0.001),miR-NC+pcDNA3.1-HMGA1组迁移细胞数明显高于miR-NC组(t=5.590,P=0.001);miR-26a mimics+pcDNA3.1-HMGA1组迁移细胞数明显高于miR-26a mimics组(t=16.046,P=0.000),低于miR-NC+pcDNA3.1-HMGA1组(t=10.400,P=0.000)。见表 3。
3 讨论在过去的几十年间,研究者[9-10]发现:miRNA可作为主要的调控因子参与肿瘤的发生,这给肿瘤分子途径的研究提供了新的视角,针对不同类型、不同时期和级别的肿瘤参与调控的miRNA可能不同,这使miRNA作为特异性肿瘤标志物成为可能。miR-26a在肿瘤方面已有很多研究,但其在肿瘤发生中的角色仍有争议[11]。研究[12-15]显示:miR-26a在肝癌和乳腺癌等肿瘤中表达水平下调,其与靶基因结合可抑制肿瘤生长;但在肺癌和胆囊癌中可通过糖原合成激酶3β(GSK-3β)使miR-26a表达水平上调,促进肿瘤的发生。miR-26a表达和生物学功能的不同可能与癌细胞遗传背景有关。miR-26a在结肠癌细胞中的作用尚未清楚,因此本研究的目的是探讨miR-26a的生物学特性。
不同肿瘤中miR-26a的作用可能不同,这与所调控的靶基因有关[16]。HMGA1可调节多个转录因子的功能,其作为癌基因在肿瘤的发生发展中起重要作用,其在正常人中一般低表达或者表达缺失,而在一些癌症患者中常过度表达[17]。研究[18]显示:HMGA1的过度表达与膀胱癌的肿瘤分期、等级、复发和预后有关,降低HMGA1的表达可阻滞细胞周期和降低细胞活性。为了验证miR-26a是否与HMGA1共同参与结肠癌的发生发展,本研究首先验证了HMGA1是否为miR-26a的靶基因,将构建的野生型和突变型HMGA1的3′-UTR荧光素酶报告载体转染至人结肠癌SW480细胞中,并通过共转染检测细胞中荧光素酶活性,结果证实HMGA1是miR-26a的靶基因。
研究[19]显示:miR-26a在肝癌中表达下调,其表达与肝癌患者的生存期及对干扰素的敏感性有关。在乳腺癌细胞和组织中miR-26a表达水平下调,将miR-26a mimics转染细胞可明显抑制细胞的生长[20]。本研究将miR-NC、miR-26a mimics和miR-26a inhibitor共转染人结肠癌SW480细胞,转染24、48和72 h后CCK8实验检测结果显示:miR-26a mimics组细胞活力明显低于miR-NC组,miR-26a inhibitor组细胞活力明显高于miR-NC组,说明miR-26a在人结肠癌发生中起重要作用。
研究[21-22]显示:HMGA基因相关的miRNA在垂体腺瘤中表达下调,进而使HMGA1和HMGA2表达上调,但在无功能的垂体腺瘤中,下调miRNA与HMGA1上调无关,说明能够靶向HMGA基因的miRNA下调可增加人垂体腺瘤中HMGA蛋白表达。研究[23-24]显示:miR-296可通过抑制HMGA1转录从而抑制HMGA1表达,当强制表达miR-296后,可通过上调HMGA1明显降低人前列腺癌细胞的增殖和侵袭,表明HMGA1可作为miRNA的靶基因对癌细胞发生发展起作用。已有研究[25-27]证实HMGA1是miR-26a的靶基因,但miR-26a是否靶向调控HMGA1对结肠癌细胞的增殖、侵袭和迁移起作用尚不清楚。本研究结果显示:miR-26a mimics组和miR-26a mimics+ pcDNA3.1-HMGA1组细胞活力、侵袭和迁移数均明显低于miR-NC组,miR-NC+pcDNA3.1-HMGA1组细胞活力、侵袭和迁移数均明显高于miR-NC组;miR-26a mimics+pcDNA3.1-HMGA1组细胞活力、侵袭和迁移数明显高于miR-26a mimics组,低于miR-NC+pcDNA3.1-HMGA1组,说明miR-26a靶向HMGA1调控结肠癌细胞的生物学特性。
综上所述,HMGA1是miR-26a的靶基因,上调miR-26a表达可抑制人结肠癌SW480细胞增殖,下调miR-26a表达可促进细胞增殖,miR-26a可靶向HMGA1抑制细胞增殖、侵袭和迁移。
| [1] | 罗真真, 张震, 乔亚敏, 等. 萝卜硫素对结直肠癌SW620细胞增殖、凋亡及迁移的影响[J]. 郑州大学学报:医学版, 2017, 52(3): 281–284. |
| [2] | Li J, Mao X, Wang X, et al. miR-433 reduces cell viability and promotes cell apoptosis by regulating MACC1 in colorectal cancer[J]. Oncol Lett, 2017, 13(1): 81–88. |
| [3] | 宋军民, 杨超, 常远, 等. miR-126在人结肠癌细胞中的表达及其对结肠癌细胞生物学行为的影响[J]. 中国老年学杂志, 2015, 35(6): 1453–1455. DOI:10.3969/j.issn.1005-9202.2015.06.007 |
| [4] | 杨泽波, 张媛媛, 陶开义, 等. miR-381在食管癌组织中的表达及临床意义[J]. 河北医学, 2018(3): 362–365. DOI:10.3969/j.issn.1006-6233.2018.03.003 |
| [5] | Sun TY, Xie HJ, He H, et al. miR-26a inhibits the proliferation of ovarian cancer cells via regulating CDC6 expression[J]. Am J Transl Res, 2016, 8(2): 1037–1046. |
| [6] | 王刚. miR-26介导EZH2调控肝癌细胞増殖及迁移的作用机制研究[D].济南: 山东大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10422-1015368007.htm |
| [7] | 林荣凯. miR-26及其靶基因HMGA1对膀胱癌的影响[D].重庆: 第三军医大学, 2015. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=D787265 |
| [8] | Akhter MZ, Rajeswari MR. Triplex forming oligonucleotides targeted to hmga1 selectively inhibit its expression and induce apoptosis in human cervical cancer[J]. J Biomol Struct Dyn, 2017, 35(4): 689–703. |
| [9] | 崔瑞新, 吕风华, 岳莹, 等. microRNA-499靶控的PI3 K/Akt通路在大鼠缺血-再灌注心肌损伤中的作用[J]. 郑州大学学报:医学版, 2016, 51(6): 740–743. |
| [10] | Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer[J]. Cancer Res, 2016, 76(13): 3666–3670. |
| [11] | Paladini L, Fabris L, Bottai G, et al. Targeting microRNAs as key modulators of tumor immune response[J]. J Exp Clin Cancer Res, 2016, 35: 103. |
| [12] | 邵青, 刘月, 宁守斌, 等. MiR-26a诱导人肝癌细胞凋亡的机制[J]. 华南国防医学杂志, 2015(10): 725–729. |
| [13] | Fkih Mhamed I, Privat M, Trimeche M, et al. miR-10b, miR-26a, miR-146a And miR-153 expression in triple negative vs non triple negative breast cancer:potential biomarkers[J]. Pathol Oncol Res, 2017, 23(4): 1–13. |
| [14] | 林高阳. miR-26a靶向GSK-3β调控β-catenin信号通路增强肺癌细胞转移潜能的机制研究[D].天津: 天津医科大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10062-1016703873.htm |
| [15] | Chandra V, Kim JJ, Mittal B, et al. MicroRNA aberrations:An emerging field for gallbladder cancer management[J]. World J Gastroenterol, 2016, 22(5): 1787–1799. DOI:10.3748/wjg.v22.i5.1787 |
| [16] | Li J, An G, Zhang M, et al. Long non-coding RNA TUG1 acts as a miR-26a sponge in human glioma cells[J]. Biochem Biophys Res Commun, 2016, 477(4): 743–748. DOI:10.1016/j.bbrc.2016.06.129 |
| [17] | Sepe R, Piscuoglio S, Quintavalle C, et al. HMGA1 overexpression is associated with a particular subset of human breast carcinomas[J]. J Clin Pathol, 2016, 69(2): 117–121. DOI:10.1136/jclinpath-2015-202907 |
| [18] | Cheng Y, Yang X, Deng X, et al. MicroRNA-218 inhibits bladder cancer cell proliferation, migration, and invasion by targeting BMI-1[J]. Tumour Biol, 2015, 36(10): 8015–8023. DOI:10.1007/s13277-015-3532-x |
| [19] | Huang FY, Wong DK, Seto WK, et al. Estradiol induces apoptosis via activation of miRNA-23a and p53:implication for gender difference in liver cancer development[J]. Oncotarget, 2015, 6(33): 34941. |
| [20] | Guzman N, Agarwal K, Asthagiri D, et al. Breast cancer-specific miR signature unique to extracellular vesicles includes "microrna-like" tRNA fragments[J]. Mol Cancer Res, 2015, 13(5): 891–901. |
| [21] | Esposito F, De Martino M, D'Angelo D, et al. HMGA1-pseudogene expression is induced in human pituitary tumors[J]. Cell Cycle, 2015, 14(9): 1471–1475. DOI:10.1080/15384101.2015.1021520 |
| [22] | Kitchen MO, Yacqub-Usman K, Emes RD, et al. Epidrug mediated re-expression of miRNA targeting the HMGA transcripts in pituitary cells[J]. Pituitary, 2015, 18(5): 674–684. |
| [23] | Zhai H, Sui M, Jiang L, et al. MiR-296 promotes colorectal cancer cells growth through regulating NF-κB[J]. Int J clinexppath, 2016, 9(4): 4391–4396. |
| [24] | Borges NM, do Vale Elias M, Fook-Alves VL, et al. Angiomirs expression profiling in diffuse large B-Cell lymphoma[J]. Oncotarget, 2016, 7(4): 4806–4816. |
| [25] | Zhong Y, Yao G, Zhao G, et al. miR-26a suppresses the growth and metastasis via targeting matrix metalloproteinase 14 in pancreatic ductal adenocarcinoma[J]. Int J Clinexppath, 2016, 9(4): 4803–4809. |
| [26] | 张玲, 屈亚威, 谈涛, 等. 高分辨率显微内镜对结肠癌诊断价值的初步研究[J]. 解放军医学杂志, 2016, 41(9): 746–749. |
| [27] | 王蓓, 林玲. 家族性高胆固醇血症临床表型及其与基因型关系[J]. 中国实用内科杂志, 2016, 36(6): 508–511. |
 2018, Vol. 44
2018, Vol. 44


