扩展功能
文章信息
- 池明, 高玲, 吴巍巍, 张博儒, 王雷
- CHI Ming, GAO Ling, WU Weiwei, ZHANG Boru, WANG Lei
- 黄连素对脂多糖诱导的小鼠急性肺损伤和炎症的改善作用及其机制
- Improvement effect of berberine on acute lung injury and inflammation induced by lipopolysaccharide and its mechanism
- 吉林大学学报(医学版), 2018, 44(06): 1194-1199
- Journal of Jilin University (Medicine Edition), 2018, 44(06): 1194-1199
- 10.13481/j.1671-587x.20180615
-
文章历史
- 收稿日期: 2018-09-17
2. 吉林大学第一医院烧伤外科, 吉林 长春 130021;
3. 吉林大学口腔医院牙周科, 吉林 长春 130021
2. Department of Burn Surgery, First Hospital, Jilin University, Changchun 130021, China;
3. Department of Periodontics, Stomatology Hospital, Jilin University, Changchun 130021, China
急性肺损伤(acute lung injury,ALI)是一种遍布全球的常见临床疾病, 其主要特征是肺内发生过度激活的炎症反应导致机体严重的气体交换功能受损,肺泡-毛细血管屏障破坏和肺部发生水肿[1-2]。内毒素(lipopolysaccharide,LPS)作为诱导ALI模型的主要物质已被广泛应用。研究[3]表明:作为革兰阴性菌外膜上发现的物质——LPS,能够激活包括肿瘤坏死因子α (tumor necrosis factor-α,TNF-α)和白细胞介素6(interleukin-6,IL-6)在内的炎症调节介质的信号级联反应,进一步加重肺组织损伤。因此控制过度的炎症反应是治疗ALI的关键。黄连素又称盐酸小檗碱,是传统的抗炎抗菌药物,可治疗肠炎和痢疾[4]。而近年来研究表明黄连素对多种疾病包括糖尿病[5]、阿尔茨海默症[6]和肿瘤[7]等具有广泛作用,但其对ALI是否具有保护作用尚未确定。因此,本研究通过构建LPS诱导的ALI模型,观察黄连素对LPS诱导的ALI的保护作用,并探讨其保护作用机制,以期为临床ALI的治疗提供依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器6~8周C57BL/6小鼠40只,购自吉林大学实验动物中心,动物合格证号:SCXK(吉)2018-0001。抗Bax、Bcl-2和GAPDH单克隆抗体购于美国Abcam公司,黄连素及HE染色相关试剂购自美国Sigma公司,超氧化物阴离子荧光探针(dihydroethidium, DHE)试剂、肺组织线粒体分离试剂和线粒体膜电位检测试剂盒[(5,5,6,6-tetrachloro-1, 1, 3, 3-tetraethylben zcmidazolylcarbocyanine iodide, (JC-1)盒)]购自上海碧云天生物技术有限公司,TNF-α和IL-6试剂盒购自于北京华英生物技术研究。酶标仪和电泳仪购自美国Bio-Rad公司。
1.2 小鼠ALI模型的制备、动物分组和给药40只小鼠随机分为对照组、LPS组、黄连素+LPS组和黄连素组,每组10只。应用无菌生理盐水配制LPS。各组小鼠分别在5 %水合氯醛麻醉,之后对照组小鼠给予生理盐水;LPS组和黄连素+LPS组小鼠将LPS(5 mg·kg-1)滴鼻给予,LPS给药后将小鼠竖立放置,缓慢旋转约15 min确保滴入的LPS能够均匀分布于两侧肺内;黄连素+LPS组小鼠在给予LPS前5天行灌胃预防性给予50mg·kg-1黄连素,每日1次,之后给予LPS滴鼻6 h;黄连素组小鼠给予50 mg·kg-1黄连素灌胃5 d,每日1次。给予LPS 6 h后,取各组小鼠支气管肺泡灌洗液(bronchoalveolar lavage fluid,BALF)和肺组织进行后续实验。
1.3 各组小鼠肺组织病理形态表现检测和肺湿干重检测肺组织石蜡切片,行常规HE染色。每组取3只小鼠,其右下肺叶采用中性甲醛液固定,石蜡包埋后进行切片,HE染色观察肺水肿程度和炎症细胞浸润,观察肺组织形态表现。将右肺上叶组织经滤纸吸去组织上的水分后称质量(湿重),置入60 ℃烘箱中72 h以上烘干至恒重,称取其质量(干重),计算肺湿干重比值。肺湿干重比值=肺湿重/肺干重。
1.4 各组小鼠BALF中总蛋白浓度和细胞渗出数检测每组取4只小鼠,5 %水合氯醛腹腔注射麻醉,暴露胸腔,小心分离气管。将4 ℃预冷的无菌PBS缓冲液0.8 mL从总支气管缓慢注入肺组织中,此时可明显看到肺部膨隆,回抽缓冲液,反复3次。将抽出的BALF收集在EP管中。将收集的BALF行1 500 r·min-1、4 ℃离心10 min,取上清进行BCA蛋白定量,计算总蛋白浓度。离心后将BALF采用50 μL 0.9 %生理盐水重悬沉淀,采用细胞计数仪计数BALF中细胞数,即为细胞渗出数。每组检测4个平行样。
1.5 各组小鼠BALF上清液中TNF-α和IL-6水平检测小鼠支气管BALF于4 ℃、12 000 r·min-1离心10 min取上清液,采用ELISA法检测BALF上清液中炎症因子TNF-α和IL-6水平,实验方法按试剂盒说明书完成。每组检测4个平行样。
1.6 各组小鼠肺组织中活性氧(reactive oxygen species, ROS)水平检测取肺组织冰冻切片,复温至室温,PBS洗涤3次,每次5 min,擦干玻片水渍,将含有0.5 μmol·L-1DHE的PBS滴加于玻片上,37 ℃孵育30 min,PBS洗涤3次,抗荧光猝灭剂封片,荧光显微镜观察肺组织中荧光强度,以荧光强度代表小鼠肺组织中ROS水平。
1.7 各组小鼠肺组织中线粒体膜电位测定按照说明书纯化和准备肺组织线粒体。将配置好的JC-1染色工作液采用JC-1染色缓冲液(1×)稀释5倍。纯化线粒体样品与5倍稀释JC-1染色工作液按1:9混合均匀,于96孔板内每孔加入100 μL,每个标本3个孔。采用荧光酶标仪检测JC-1单体时激发波长为490 nm,发射波长为530 nm;检测JC-1聚合物时激发波长为525 nm,发射波长为590 nm。将得到单体吸光度(A1)值与聚合物的吸光度(A2)值进行比较。如果A1值升高而A2值降低即代表线粒体膜电位降低,线粒体损伤。
1.8 Western blotting法检测各组小鼠肺组织中Bax/Bcl-2比值将小鼠肺组织剪碎、匀浆和裂解,4 ℃、12 000 r·min-1离心15 min。采用BIO-RAD法测定蛋白表达水平。取50 μg蛋白行SDS-PAGE电泳,将蛋白样本转移至PVDF膜上。取出膜后采用5%脱脂奶粉封闭1 h,PBST洗涤3次,加入抗Bax抗体(1:500)和Bcl-2 (1:1 000),4 ℃孵育过夜。次日,PBST洗涤3次,加入相应过氧化物酶标记的二抗,置于脱色摇床摇1 h,PBST洗涤3次。以Bio-Rad凝胶成像系统照相并分析。以GAPDH作为内参蛋白,实验重复3次。Bax和Bcl-2蛋白表达水平=每个样本条带灰度值/GAPDH灰度值。计算Bax/Bcl-2比值。Bax/Bcl-2=Bax蛋白表达水平/Bcl-2蛋白表达水平。
1.9 统计学分析采用SPSS 15.0统计软件进行统计学分析。各组小鼠肺湿干重比值、BALF中总蛋白浓度、BALF中细胞渗出数、BALF上清液中TNF-α和IL-6水平、肺组织中线粒体膜电位、肺组织中Bcl-2和Bax蛋白表达水平,以及Bax/Bcl-2比值以x±s表示,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠肺组织形态表现与对照组比较,LPS组小鼠炎症细胞浸润肺间质和肺泡空间增加,肺泡壁明显增厚;与LPS组比较,黄连素组小鼠肺组织病理损害明显减轻;黄连素组和对照组小鼠肺组织病理形态无明显区别。见图 1(插页四)。
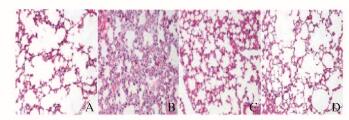
|
| A:Control group; B:LPS group; C:Berberine+LPS group; D:Berberine group. 图 1 各组小鼠肺组织形态表现(HE,×200) Figure 1 Morphology of lung tissue of mice in various groups (HE, ×200) |
|
|
与对照组比较,LPS组小鼠肺湿干重比值明显升高(P < 0.01);与LPS组比较,黄连素+LPS组小鼠肺湿干重比值降低(P < 0.05);黄连素组小鼠肺湿干重比值与对照组比较差异无统计学意义(P>0.05)。见图 2。
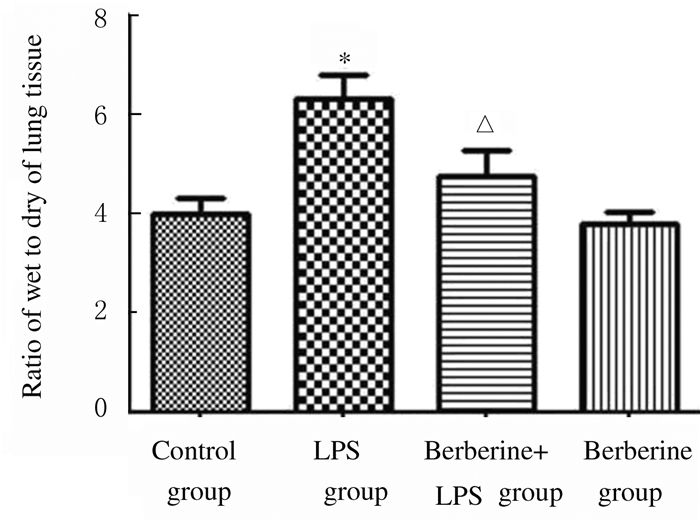
|
| *P < 0.01 vs control group; △P < 0.05 vs LPS group. 图 2 各组小鼠肺湿干重比值 Figure 2 Ratios of wet to dry weights of lung tissue of mice in various groups |
|
|
与对照组比较,LPS组小鼠BALF中总蛋白浓度升高(P < 0.05);与LPS组比较,黄连素+LPS组小鼠BALF中总蛋白浓度明显降低(P < 0.05),见图 3。与对照组比较,LPS组小鼠BALF中细胞渗出数增加(P < 0.01);与LPS组比较,黄连素+LPS组小鼠BALF中细胞渗出数降低(P < 0.05),见图 4。
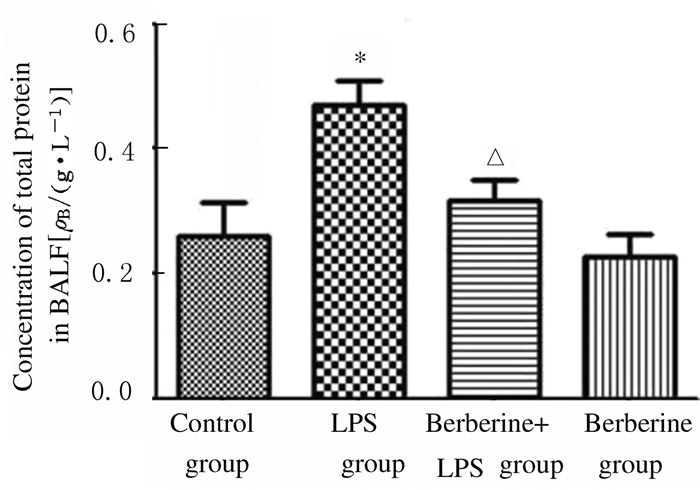
|
| *P < 0.05 vs control group; △P < 0.05 vs LPS group. 图 3 各组小鼠BALF中总蛋白浓度 Figure 3 Concentrations of total protein in BALF of mice in various groups |
|
|
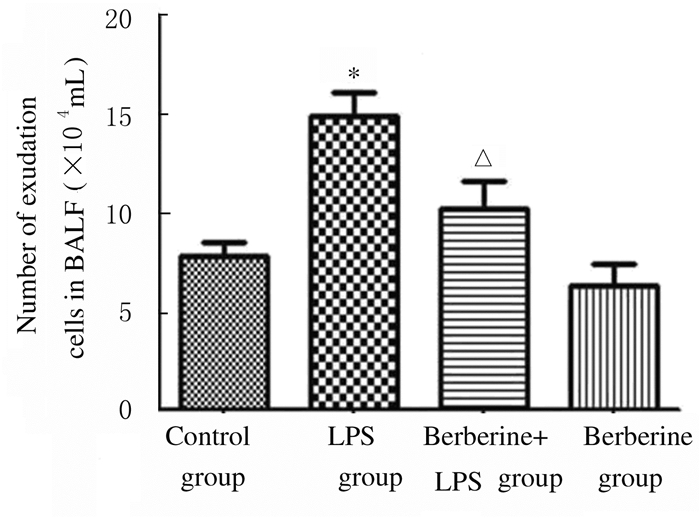
|
| * P < 0.01 vs control group; △P < 0.05 vs LPS group. 图 4 各组小鼠BALF中细胞渗出数 Figure 4 Number of exudation cells in BALF of mice in various groups |
|
|
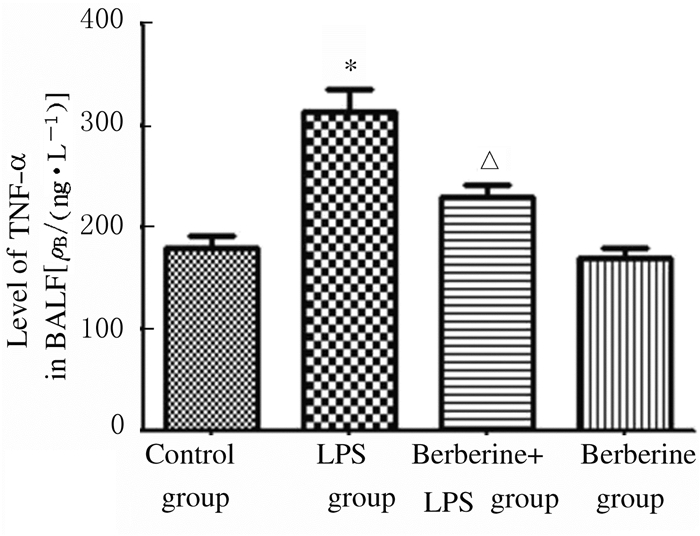
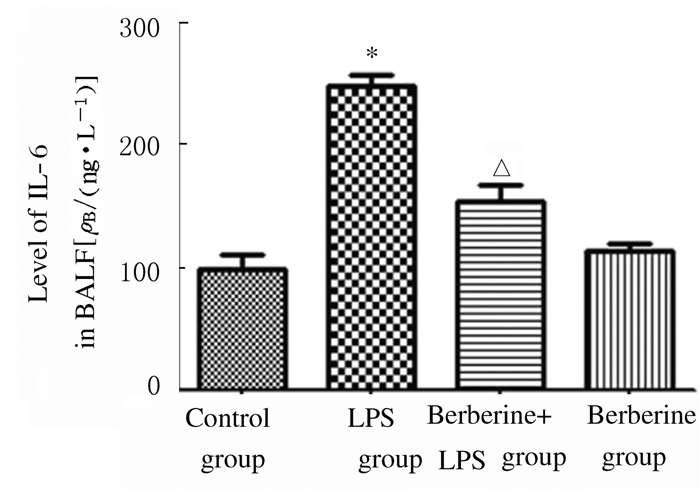
与对照组比较,LPS组小鼠BALF上清液中TNF-α和IL-6水平升高(P < 0.01);与LPS组比较,黄连素+LPS组小鼠BALF上清液中TNF-α和IL-6水平降低(P < 0.01);与对照组比较,黄连素组小鼠BALF上清液中TNF-α和IL-6水平差异无统计学意义(P>0.05)。见图 5和6。
|
| * P < 0.01 vs control group; △P < 0.01 vs LPS group. 图 5 各组小鼠BALF中TNF-α水平 Figure 5 Levels of TNF-α in BALF of mice in various groups |
|
|
|
| *P < 0.01 vs control group; △P < 0.01 vs LPS group. 图 6 各组小鼠BALF中IL-6水平 Figure 6 Levels of IL-6 in BALF of mice in various groups |
|
|
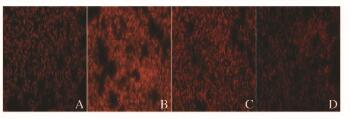
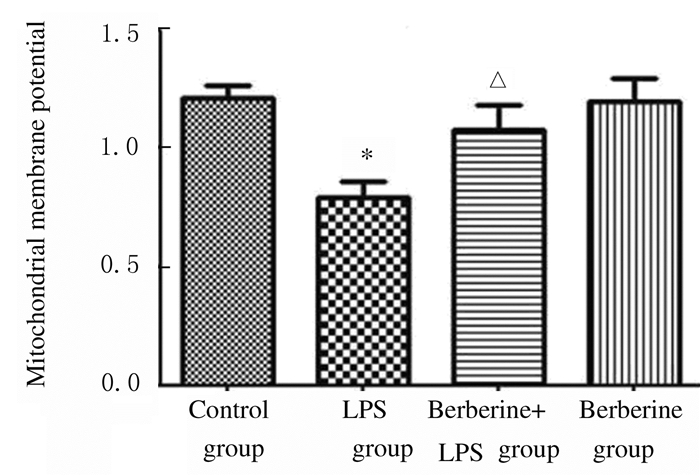
正常情况下细胞中产生低水平ROS,LPS组小鼠肺组织中ROS水平升高;与LPS组比较,黄连素+LPS组小鼠肺组织中ROS水平降低(图 7,见插页四)。与对照组比较,LPS组小鼠肺组织中线粒体膜电位降低(P < 0.01);与LPS组比较,黄连素+LPS组小鼠肺组织中线粒体膜电位升高(P < 0.05)。见图 8。
|
| A: Control group; B: LPS group; C: Berberine+LPS group; D: Berberine group. 图 7 各组小鼠肺组织中超氧化物阴离子的荧光强度(×200) Figure 7 Fluorescence intensities of superoxide anion in lung tissue of mice in various groups (×200) |
|
|
|
| * P < 0.01 vs control group; △P < 0.05 vs LPS group. 图 8 各组小鼠肺组织中线粒体膜电位 Figure 8 Mitochondrial membrane potential in lung tissue of mice in various groups |
|
|
与对照组比较,LPS组小鼠肺组织中Bax蛋白表达水平升高,Bcl-2蛋白表达水平降低,Bax/Bcl-2比值升高(P < 0.01);与LPS组比较,黄连素+LPS组小鼠肺组织中Bax蛋白表达水平降低,Bcl-2蛋白表达水平升高,Bax/Bcl-2比值降低(P < 0.01)。见图 9和10。
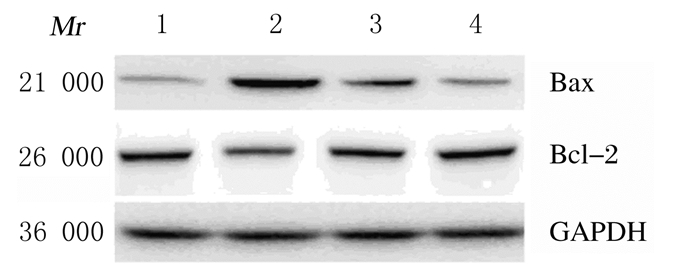
|
| Lane 1:Control group; Lane 2:LPS group; Lane 3:Berberine+LPS group; Lane 4:Berberine group. 图 9 各组小鼠肺组织中Bax和Bcl-2蛋白表达电泳图 Figure 9 Electrophoregram of expressions of Bax and Bcl-2 proteins in lung tissue of mice in various groups |
|
|
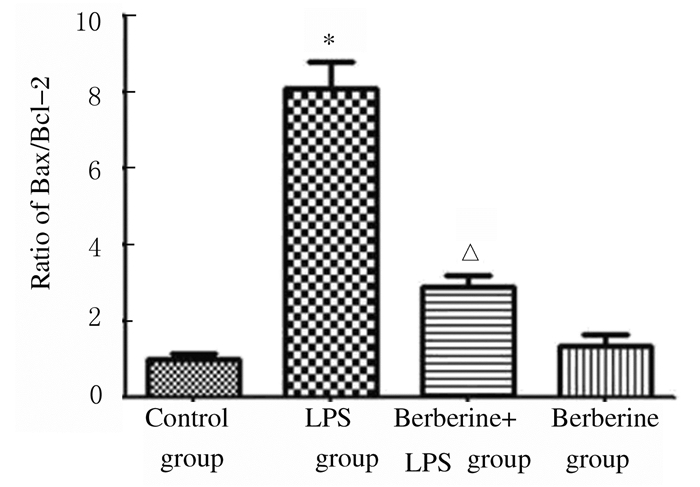
|
| *P < 0.01 vs control group; △P < 0.01 vs LPS group. 图 10 各组小鼠肺组织中Bax/Bcl-2比值 Figure 10 Ratio of Bax /Bcl-2 in lung tissue of mice in various groups |
|
|
ALI/急性呼吸窘迫综合征(ARDS)是临床常见的危急重症,发病机制复杂,目前尚未完全阐明,病情发展迅速,病死率高。LPS是革兰阴性菌的主要致病成分[7],是引起ALI并最终导致患者死亡的重要原因之一,LPS刺激下,炎症细胞迅速浸润肺组织,引起中性粒细胞和巨噬细胞浸润等炎症细胞聚集[8-9],进而导致相关炎性细胞激活,释放IL-1β、IL-6和TNF-α等一系列炎症介质[10],造成肺组织损伤。本研究结果显示:LPS可引起明显的肺组织炎症、肺泡间隔增厚、间质充血、水肿和BALF中炎性细胞增多。因此,控制炎症是治疗ALI的关键环节。
黄连素又名小檗碱,是自毛莨科黄连属植物黄连的根状茎中提取的主要有效成分,黄连素具有抗菌、抗炎和抗病毒等多种药理作用[11-12]。研究[13]表明:黄连素可以通过激活磷酸腺苷活化的蛋白激酶(adenosine monophosphate-activated protein kinase, AMPK)和转录因子3(transcription factor 3, ATF-3)抑制LPS诱导巨噬细胞中炎症因子IL-6、TNF-α和IL-1β的释放,降低炎症反应程度。在大鼠哮喘模型中,黄连素对卵清蛋白诱导的气道炎症具有明显的抑制作用,其机制与抑制NF-κB信号途径有关[14]。因此本研究采用黄连素治疗ALI具有一定可行性。本研究结果表明:提前5 d连续给予黄连素后,小鼠肺组织炎症反应程度明显降低,气道壁增厚,肺水肿的严重程度亦降低。同时,黄连素明显改善了LPS引起BALF中细胞渗出数增多和TNF-α及IL-6的水平升高。由此可见,黄连素对LPS诱导的肺损伤和炎症具有明显的保护作用。
氧化应激是ALI中炎症发生发展的重要机制之一,ROS与多种细胞信号传导级联有关,是导致呼吸道炎症的重要介质[15-17]。研究[18]表明应用芦荟苷通过抑制ROS产生可明显降低LPS诱导的肺损伤程度。研究[19]显示青蒿琥酯可降低ROS诱导的NOD样受体家族蛋白(NOD-like receptor protein, NLRP3)炎性小体激活从而改善缺血再灌注介导的肺组织损伤。研究[20]表明:黄连素通过抗氧化功能保护巨细胞病毒引起的螺旋神经元的凋亡。在糖尿病神经病实验中,黄连素能降低氧化还原反应失衡,保护线粒体功能,降低高糖诱导的神经元损伤[21]。DHE是最常用的检测细胞中超氧化物阴离子水平的荧光探针,可用来检测细胞中ROS水平,其发光强度与ROS水平呈正相关关系。黄连素抑制LPS诱导肺损伤和炎症是否与抑制ROS保护线粒体功能有关目前尚未明确。本研究结果表明:在LPS诱导的ALI模型中,ROS被激活,进而引起线粒体损伤和凋亡。黄连素通过降低肺损伤小鼠模型中ROS的产生,改善了线粒体膜电位水平的降低趋势,降低了Bax/Bcl-2比值,进而抑制了LPS诱导肺损伤组织中的细胞凋亡。可见黄连素可能通过降低LPS引起的氧化应激保护LPS诱导的肺损伤及炎症,其机制与保护线粒体功能和减少线粒体凋亡有关。
综上所述,黄连素明显改善了LPS诱导的肺组织损伤和炎症的程度,其机制可能与抑制体内氧化应激和稳定线粒体功能有关。
| [1] | Amin Z, Rahmawati FN. Recent insight into potential acute respiratory distress syndrome[J]. Saudi Med J, 2017, 38(4): 344–349. DOI:10.15537/smj.2017.4.15843 |
| [2] | Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report:features and measurements of experimental acute lung injury in animals[J]. Am J Respir Cell Mol Biol, 2011, 44(5): 725–738. DOI:10.1165/rcmb.2009-0210ST |
| [3] | Lee JW, Chun W, Kwon OK, et al. 3, 4, 5-Trihydroxycinnamic acid attenuates lipopolysaccharide (LPS)-induced acute lung injury via downregulating inflammatory molecules and upregulating Ho-1/Ampk activation[J]. Int Immunopharmacol, 2018, 64: 123–130. DOI:10.1016/j.intimp.2018.08.015 |
| [4] | Yu XT, Xu YF, Huang YF, et al. Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice[J]. PLoS One, 2018, 13(3): e0194069. DOI:10.1371/journal.pone.0194069 |
| [5] | Zhu L, Han J, Yuan R, et al. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway[J]. Biol Res, 2018, 51(1): 9. DOI:10.1186/s40659-018-0157-8 |
| [6] | Hussien HM, Abd-Elmegied A, Ghareeb DA, et al. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer's-like disease in rats[J]. Food Chem Toxicol, 2018, 111: 432–444. DOI:10.1016/j.fct.2017.11.025 |
| [7] | Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine[J]. Expert Rev Respir Med, 2010, 4(6): 773–783. DOI:10.1586/ers.10.71 |
| [8] | Yu P, Dong L, Zhang Y, et al. Design, synthesis and biological activity of novel asymmetric C66 analogs as anti-inflammatory agents for the treatment of acute lung injury[J]. Eur J Med Chem, 2015, 94: 436–446. DOI:10.1016/j.ejmech.2014.11.054 |
| [9] | 刘笑玎, 原铭贞, 王司仪, 等. 水通道蛋白1、3、4和5与急性肺损伤关系的研究进展[J]. 吉林大学学报:医学版, 2014, 40(5): 1119–1122. |
| [10] | Chen J, Wang JB, Yu CH, et al. Total flavonoids of mosla scabra leaves attenuates lipopolysaccharide-induced acute lung injury via down-regulation of inflammatory signaling in mice[J]. J Ethnopharmacol, 2013, 148(3): 835–841. DOI:10.1016/j.jep.2013.05.020 |
| [11] | 余园媛, 王伯初, 彭亮, 等. 黄连的药理研究进展[J]. 重庆大学学报, 2006, 29(2): 107–111. |
| [12] | 赵立峰, 李明. 黄连素研究进展[J]. 唐山学院学报, 2008, 21(6): 35–36. DOI:10.3969/j.issn.1672-349X.2008.06.011 |
| [13] | Bae YA, Cheon HG. Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages[J]. Korean J Physiol Pharmacol, 2016, 20(4): 415–424. DOI:10.4196/kjpp.2016.20.4.415 |
| [14] | Li Z, Zheng J, Zhang N, et al. Berberine improves airway inflammation and inhibits NF-κB signaling pathway in an ovalbumin-induced rat model of asthma[J]. J Asthma, 2016, 53(10): 999–1005. DOI:10.1080/02770903.2016.1180530 |
| [15] | 张静, 马翠丽, 王志国. 黄芪甲苷对大鼠心肌局部缺血再灌注损伤的改善作用及其PI3K/Akt/mTOR信号通路的影响[J]. 吉林大学学报:医学版, 2014, 40(5): 991–996. |
| [16] | 冉琴, 张雷, 张沄, 等. 白藜芦醇对中性粒细胞性哮喘小鼠肺组织炎症的抑制作用及其机制[J]. 吉林大学学报:医学版, 2018, 44(5): 897–902. |
| [17] | Boukhenouna S, Wilson MA, Bahmed K, et al. Reactive oxygen species in chronic obstructive pulmonary disease[J]. Oxid Med Cell Longev, 2018, 2018: 5730395. |
| [18] | Jiang K, Guo S, Yang C, et al. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway[J]. Int Immunopharmacol, 2018, 64: 140–150. DOI:10.1016/j.intimp.2018.08.023 |
| [19] | Liu Z, Qu M, Yu L, et al. Artesunate inhibits renal ischemia-reperfusion-mediated remote lung inflammation through attenuating ROS-induced activation of NLRP3 inflammasome[J]. Inflammation, 2018, 41(4): 1546–1556. DOI:10.1007/s10753-018-0801-z |
| [20] | Zhuang W, Li T, Wang C, et al. Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis[J]. Free Radic Biol Med, 2018, 121: 127–135. DOI:10.1016/j.freeradbiomed.2018.04.575 |
| [21] | Yerra VG, Kalvala AK, Sherkhane B, et al. Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy[J]. Neuropharmacology, 2018, 131: 256–270. DOI:10.1016/j.neuropharm.2017.12.029 |
 2018, Vol. 44
2018, Vol. 44


