扩展功能
文章信息
- 赵菁, 刘剑, 张敏, 吴珊, 赵本正, 陈俊宇, 金延泽
- ZHAO Jing, LIU Jian, ZHANG Min, WU Shan, ZHAO Benzheng, CHEN Junyu, JIN Yanze
- 除草剂阿特拉津的免疫毒性及其对大鼠免疫功能的影响
- Immunotoxicity of herbicide atrazine and its effect on immune function of rats
- 吉林大学学报(医学版), 2018, 44(06): 1163-1168
- Journal of Jilin University (Medicine Edition), 2018, 44(06): 1163-1168
- 10.13481/j.1671-587x.20180609
-
文章历史
- 收稿日期: 2018-08-07
2. 吉林大学第二医院妇产科, 吉林长春 130041;
3. 吉林大学第二医院病理科, 吉林长春 130041
2. Department of Obstetrics and Gynecology, Second Hospital, Jilin University, Changchun 130041, China;
3. Department of Pathology, Second Hospital, Jilin University, Changchun 130041, China
阿特拉津(atrazine, ATR)是世界上使用最广泛的除草剂之一[1],在土壤和地表水中持续存在并具有生物蓄积作用,可能对人类健康构成重大威胁[2]。免疫系统有识别自己和异己的功能,当某些因素导致机体免疫功能失调时,常会诱发各种疾病。已有研究[3-4]表明:长时间暴露于ATR的鱼类及两栖动物免疫功能受到抑制。Nikolay等[5]研究显示:接触ATR 14 d后C57BL/6成年小鼠的胸腺质量和脾质量减少,构成细胞持续减少。流行病学研究[6]显示:ATR暴露与肿瘤的发生发展密切相关,其可能是生殖系统肿瘤的危险因子。目前关于ATR亚急性暴露对大鼠免疫功能的影响尚需进一步研究,本实验通过探讨ATR对健康大鼠免疫功能的影响,从免疫学角度阐明ATR导致机体损伤的机制,从而为全面评价ATR毒性效应及风险评估提供科学依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器4周龄Wistar雌性大鼠32只,购于吉林大学基础医学院实验动物中心(动物合格证号:201600014675),饲养于恒定温度(22℃~25℃)、恒定湿度(40%~50%)环境中,标准饲料和水供动物自由食用。ATR(纯度为98.99%,以橄榄油溶解)和刀豆蛋白A(concanavalin A, Con A)购于美国Sigma公司,RPMI 1640和胎牛血清购自美国Gibco公司,白细胞介素1(IL-1)和白细胞介素6 (IL-6)酶联免疫试剂盒购于美国R&D公司,CD3和CD8荧光标记抗体购于美国Ebioscience公司,以橄榄油溶解ATR并制备0.5、2.5和12.5 g·L-1ATR溶液(4℃条件下最多保存1周)。BIO-RAD550酶标仪购于美国Biorad公司,YZ-875超净工作台购于苏州净化设备厂,自动CO2恒温培养箱购于日本ANYO公司,101A-2电热鼓风干燥箱购于上海实验仪器总厂,ss-245自动高压蒸气消毒器购于日本SONY公司。
1.2 动物分组和处理32只Wistar雌性大鼠随机分为4组,每组8只。对照组大鼠以等体积橄榄油灌胃,低、中、高剂量ATR组大鼠分别给予5、25和125 mg·kg-1ATR灌胃,每日1次,连续给药28 d,第29天处死各组大鼠,取血及脾脏备用。
1.3 各组大鼠体质量和脾质量的测定大鼠于ATR开始给药当天和其后每7天称体质量,第29天处死大鼠,脾脏无菌称质量,计算脾指数。脾指数=脾质量(mg)/体质量(g)。
1.4 脾单细胞悬液的制备无菌取各组大鼠的脾组织,剪碎,置于无菌尼龙网上,滴入RPMI 1640完全培养液,轻轻研磨,采用400 μm不锈钢网去除细胞碎片。采用低渗缓冲溶液裂解红细胞,以淋巴细胞分离液密度梯度离心法(400 g、20 min)分离脾淋巴细胞,PBS洗涤并重悬于RPMI1640培养基(含有10%FBS、100 U·mL-1青霉素、100 mg·L-1链霉素和2 mmol·L-1L-谷氨酰胺)中,采用台盼蓝排除法检测细胞活力,当细胞活力>95%时进行细胞计数。
1.5 HE染色检测各组大鼠脾组织病理形态表现收集脾脏,固定于10%甲醛缓冲液中,石蜡包埋,然后切片。切片采用苏木精溶素染色用于组织学评估和镜检。
1.6 淋巴细胞转化实验检测各组大鼠脾淋巴细胞转化率调整淋巴细胞浓度至2×107mL-1,接种于96孔培养板,每孔100 μL培养过夜。实验孔更换为含40 mg·L-1ConA的RPMI 1460培养液,对照孔更换为RPMI 1640培养液,设3个重复孔,置于37℃、5%CO2恒温孵箱中无菌培养48 h。于分光光度仪490 nm处读取吸光度(A)值,计算脾淋巴细胞转化率。脾淋巴细胞转化率=实验孔A值/对照孔A值×100%。
1.7 各组大鼠NK细胞杀伤活性的检测如上所述制备来自小鼠脾脏的效应细胞。NK细胞对小鼠淋巴瘤细胞(即YAC-1细胞)敏感,因此采用YAC-1细胞作为靶细胞。将1×105 L-1YAC-1细胞和效应细胞分别加入96孔板中。孵育48 h后采用分光光度仪于490 nm处读取A值,计算NK细胞杀伤活性。NK细胞杀伤活性= 1-(E+T的A值- E的A值)/ T的A值×100%,其中E+T=效应细胞和靶细胞的混合物,E=效应细胞,T=靶细胞。
1.8 流式细胞术检测各组大鼠脾细胞中CD3+和CD8+ T细胞百分比脾细胞重悬于100 μL结合缓冲液,加入10 μLCD3 PE-Cy5或CD8 PE抗体,37℃孵育15 min后PBS洗涤3次,随后于流式细胞仪上测定,以对照组为参照,通过CELL Quest软件分析并以侧向和正向散射特征定义细胞亚型,分析CD3+和CD8+ T细胞所占百分比。
1.9 ELISA法测定各组大鼠血清中IL-1和IL-6水平处死各组大鼠,收集血液,3 000 g×10 min离心收集血清,按照ELISA试剂盒说明书操作,测定各组大鼠血清中IL-1和IL-6水平。
1.10 统计学分析采用SPSS 17.0统计软件对数据进行统计学分析。各组大鼠体质量、脾质量、淋巴细胞转化率、NK细胞杀伤活性、CD3+和CD8+T细胞百分比、血清中IL-1和IL-6水平以x±s表示,组间比较采用独立样本t检验,组内两两比较采用单因素方差分析。以P<0.05为差异有统计学意义。
2 结果 2.1 各组大鼠体质量本实验中,所有大鼠均存活到实验结束。与对照组比较,各剂量ATR组大鼠体质量差异无统计学意义(P>0.05);各剂量ATR组大鼠体质量组间比较差异无统计学意义(P>0.05)。见表 1。
| (n=8, x±s) | |||
| Group | Dose (mg·kg-1) |
Body weight (m/g) |
Spleen index [mB/(mg·g-1)] |
| Control | 0 | 170.43±19.43 | 33.77±2.15 |
| ATR | |||
| Low dose | 5 | 174.00±13.71 | 31.00±2.75 |
| Middle dose | 25 | 172.57±21.13 | 32.32±2.77 |
| High dose | 125 | 159.57±18.91 | 29.88±3.55* |
| * P<0.05 compared with control group. | |||
与对照组比较,低和中剂量ATR组大鼠脾指数差异无统计学意义(P>0.05),高剂量ATR组大鼠脾指数降低(P<0.05);各剂量ATR组大鼠脾指数组间比较差异无统计学意义(P>0.05)。见表 1。
2.3 各组大鼠脾组织病理形态表现与对照组比较,低剂量ATR组大鼠脾组织无明显变化;中剂量ATR组大鼠脾组织生发中心略减少;高剂量ATR组大鼠脾组织呈现明显退行性变,生发中心消失,白髓减少,红髓充血。说明高剂量ATR组大鼠脾脏产生了毒性反应。见图 1(插页三)。
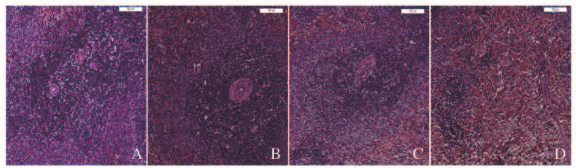
|
| A: Control group;B:Low dose of ATR group;C:Middle dose of ATR group;D:High dose of ATR group. 图 1 各组大鼠脾脏组织形态表现(HE,×100) Figure 1 Morphology of spleen tissue of rats in various groups (HE, ×100) |
|
|
与对照组比较,低和中剂量ATR组大鼠脾淋巴细胞转化率差异无统计学意义(P>0.05)。高剂量ATR组大鼠脾淋巴细胞转化率明显降低(P<0.05)。见表 2。
| (n=8, x±s.η/%) | |||||
| Group | Dose(mg·kg-1) | Lymphocyte transformation rate |
Killing activity of NK cells |
CD3+ T cells | CD8+ T cells |
| Control | 0 | 1.71±0.13 | 59.67±5.18 | 10.34±1.89 | 11.89±1.90 |
| ATR | |||||
| Low dose | 5 | 1.55±0.31 | 53.39±6.59 | 9.31±1.89 | 9.47±1.74 |
| Middle dose | 25 | 1.43±0.16 | 53.49±5.57 | 8.66±1.46 | 9.21±1.55 |
| High dose | 125 | 1.39±0.10* | 49.49±6.34 | 6.21±1.21* | 7.62±1.80* |
| * P<0.05 compared with control group. | |||||
与对照组比较,各剂量ATR组大鼠脾细胞中NK细胞杀伤活性差异无统计学意义(P>0.05)。见表 2。
2.6 各组大鼠脾细胞中CD3+和CD8+T细胞百分比与对照组比较,低和中剂量ATR组大鼠脾细胞CD3+和CD8+T细胞百分比差异无统计学意义(P>0.05),高剂量ATR组大鼠脾细胞中CD3+T细胞和CD8+T细胞百分比降低(P<0.05);各剂量ATR组大鼠脾细胞CD3+和CD8+T细胞亚群百分比比较差异无统计学意义(P>0.05)。见表 2。
2.7 各组大鼠血清中IL-1和IL-6水平与对照组比较,低和中剂量ATR组大鼠血清中IL-1和IL-6水平差异无统计学意义(P<0.05),高剂量ATR组大鼠血清中IL-1和IL-6水平升高(P<0.05);各剂量ATR组大鼠血清中IL-1和IL-6水平比较差异无统计学意义(P>0.05)。见表 3。
| [n=8, x±s, ρB/(ng·L-1)] | |||
| Group | Dose (mg·kg-1) |
IL-1 | IL-6 |
| Control | 0 | 660.06±127.86 | 378.00±86.09 |
| ATR | |||
| Low dose | 5 | 672.84±138.53 | 423.93±42.74 |
| Middle dose | 25 | 670.51±107.42 | 417.82±27.04 |
| High dose | 125 | 754.83±130.31* | 467.38±37.03* |
| * P<0.05 compared with control group. | |||
ATR是目前世界上应用最广的除草剂之一,其广泛应用所引起的环境问题已引起了人们的关注[7]。尽管目前欧盟已经禁止使用ATR,但是中国和美国仍然大量使用ATR[8]。研究[9]显示:ATR可导致Wistar大鼠运动减少,自发性肌肉活动和全身僵硬症。研究[10-14]显示:长期与ATR接触会对心脏、肺、血液、神经及生殖系统造成损伤,甚至导致癌症。已有研究[15-17]证实:ATR可引起生物体免疫功能抑制。Chen等[15]研究显示:ATR对小鼠细胞免疫、体液免疫和非特异性免疫功能具有抑制作用,Zhao等[16]研究显示:ATR亚急性暴露影响小鼠脾细胞功能。Karrowa等[17]发现ATR可降低雌性B6C3F1小鼠的细胞免疫及机体抗病能力。Nikolay等[5]发现:C57BL/6成年小鼠连续暴露于250 mg·kg -1ATR条件下14 d后,脾脏构成细胞持续减少,树突状细胞的成熟被抑制,胸腺中CD4+/CD8+T细胞受明显影响。ATR通过抑制脾脏树突状细胞成熟、干扰体液和细胞介导的免疫反应,由此可说明ATR处理后小鼠对肿瘤的宿主抵抗力减弱的原因[18]。Pinchuk等[19]发现ATR干扰树突状细胞成熟从而清除组织相容性抗原Ⅰ(MHC-Ⅰ)分子,ATR可能有助于免疫逃避。Karrow等[20]发现:ATR抑制小鼠胸腺指数和巨噬细胞数,减少脾CD8+细胞数和杀伤性细胞数,并剂量依赖性地降低小鼠对B16F10黑色素瘤的宿主抵抗力。可见ATR可能通过免疫抑制降低肿瘤耐受力。
本研究结果显示:与对照组比较,各剂量ATR组中大鼠体质量比较差异无统计学意义,但高剂量ATR组中大鼠脾指数降低。HE染色结果提示:高剂量ATR组大鼠脾脏出现明显退行性变,具体表现为生发中心消失,白髓减少,红髓充血,说明125 mg·kg-1ATR组可对大鼠脾脏产生毒性反应。
CD3+T淋巴细胞是外周血中代表成熟T淋巴细胞的标志物之一,本研究结果显示:与对照组比较,高剂量ATR组大鼠脾细胞中CD3+和CD8+T细胞百分比降低,提示ATR可影响脾脏中T淋巴细胞亚群的分布,减少CD3+T淋巴细胞的亚群百分比,破坏效应T淋巴细胞亚群的构成平衡和细胞免疫功能的稳定。淋巴细胞转化率是反映机体细胞免疫的基本指标[21],本实验中大鼠淋巴细胞转化实验结果显示:与对照组比较,高剂量ATR组大鼠脾淋巴细胞转化率降低,提示ATR有抑制T细胞免疫功能的作用。以上改变可能有利于肿瘤免疫耐受的形成,降低免疫系统对肿瘤免疫的应答。Lee等[22]发现:ATR促使脾脏CD3+T细胞亚群减少,而CD19+B淋巴细胞和非淋巴细胞未受影响,并进一步探讨其机制发现ATR触发了半胱氨酸天冬氨酸蛋白酶3(caspase-3)的激活和半胱氨酸天冬氨酸蛋白酶8(caspase-8)和聚腺苷二磷酸-核糖聚合酶(poly-ADP-ribose polymerase,PARP)的裂解,进而诱导内质网应激介导的T淋巴细胞凋亡。
白细胞介素是由白细胞和其他细胞产生,在细胞间发挥作用的细胞因子。细胞因子是监测动物和人类免疫系统的一个敏感指标,Xang等[23]发现:将幼年鲤鱼暴露于高浓度ATR下,其脾组织中IL-1表达水平明显升高。本研究结果显示:ATR暴露后,与对照组比较,高剂量ATR组大鼠血清中IL-1和IL-6水平均升高。IL-1的分泌可刺激免疫细胞产生IL-6,IL-6过度表达与肿瘤进展失调相关,其机制与多种信号通路特别是信号转导和转录激活因子3(Stat3)信号通路相关。研究[24]显示:患者血清中IL-6水平升高与各种癌症(如多发性骨髓瘤、非小细胞肺癌、结直肠癌、肾细胞癌、前列腺癌、乳腺癌和卵巢癌)相关,且与患者生存期缩短有关。因此ATR可能通过提高血清中IL-1水平,促进IL-6分泌,进而通过IL-6激活多信号通路,最终对肿瘤的发生发展起到促进作用。
综上所述,ATR作为一种环境污染物,具有脾脏毒性,能够降低淋巴细胞转化率,减少CD3+和CD8+T细胞数量,可能有利于肿瘤免疫耐受的形成进而影响机体的抗肿瘤能力;同时ATR通过提高IL-1水平,促进IL-6分泌,进而激活多条信号通路,从而对肿瘤的发生发展起促进作用。因此ATR的免疫毒性应该引起足够的重视。
| [1] | Mackul'ak T, Prousek J, Svorc L. Degradation of atrazine by Fenton and modified Fenton reactions[J]. Monatshefte Fur Chemie, 2011, 142(6): 561–567. DOI:10.1007/s00706-011-0504-8 |
| [2] | Lasserre JP, Fack F, Revets D, et al. Effects of the endocrine disruptors atrazine and PCB 153 on the protein expression of MCF-7 human cells[J]. J Proteome Res, 2009, 8(12): 5485–5496. DOI:10.1021/pr900480f |
| [3] | Khalil SR, Reda RM, Awad A. Efficacy of spirulina platensis diet supplements on disease resistance and immune-related gene expression in Cyprinus carpio L. exposed to herbicide atrazine[J]. Fish Shellfish Immunol, 2017, 67: 119–128. DOI:10.1016/j.fsi.2017.05.065 |
| [4] | Rohr JR, Mccoy KA. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians[J]. Environ Health Perspect, 2010, 118(1): 20–32. DOI:10.1289/ehp.0901164 |
| [5] | Nikolay MF, Lesya MP, Bobbie BL, et al. Immunotoxic effects of short-term atrazine exposure in young male C57BL/6Mice[J]. Toxicol Sci, 2005, 86(2): 324–332. DOI:10.1093/toxsci/kfi188 |
| [6] | Simpkins JW, Swenberg JA, Weiss N, et al. Atrazine and breast cancer:a framework assessment of the toxicological and epidemiological evidence[J]. Toxicol Sci, 2011, 123(2): 441–459. DOI:10.1093/toxsci/kfr176 |
| [7] | Rodrigues ET, Alpendurada MF, Ramos F, et al. Environmental and human health risk indicators for agricultural pesticides in estuaries[J]. Ecotoxicol Environ Saf, 2018, 150: 224–231. DOI:10.1016/j.ecoenv.2017.12.047 |
| [8] | Sass JB, Colangelo A. European Union bans atrazine, while the United States negotiates continued use[J]. Int J Occup Environ Health, 2006, 12(3): 260–267. DOI:10.1179/oeh.2006.12.3.260 |
| [9] | Kale OE, Oyesola TO, Raji FS. Celecoxib, a cyclooxygenase-2 inhibitor, offers chemoprevention against reproductive and neurobehavioural abnormalities induced by atrazine in male Wistar rats[J]. Environment Toxicol Pharmacol, 2018, 58: 84–97. DOI:10.1016/j.etap.2017.12.026 |
| [10] | Graziano N, Mcguire MJ, Roberson A, et al. 2004 national atrazine occurrence monitoring program using the abraxis ELISA method[J]. Environment Sci Technol, 2006, 40(4): 1163–1171. DOI:10.1021/es051586y |
| [11] | Li XN, Zuo YZ, Qin L, et al. Atrazine-xenobiotic nuclear receptor interactions induce cardiac inflammation and endoplasmic reticulum stress in quail[J]. Chemosphere, 2018, 206: 549–559. DOI:10.1016/j.chemosphere.2018.05.049 |
| [12] | Belloni V, Alleva E, Dessi-Fulgheri F, et al. Effects of low doses of atrazine on the neurobehacioural development of mice[J]. Ethol Ecol Evilut, 2007, 19(4): 309–322. DOI:10.1080/08927014.2007.9522554 |
| [13] | Shibayama H, Kotera T, Shinoda Y, et al. Collaborative work on ealuation of ovariantoxicity 14 two-or four-week repeated-dose studies and fertilitystudy of atrazine in female rats[J]. J Toxicolog Sci, 2009, 34: 3. |
| [14] | Ueda M, Imai T, Takizawa T, et al. Possible enhancing effects of atrazine on growth of 7, 12-dimethylbenz(a)anthracene-induced mamary tumors inovariectomized Sprague-Dawleyrats[J]. Cancer Sci, 2005, 96(1): 19–25. DOI:10.1111/j.1349-7006.2005.00008.x |
| [15] | Chen JY, Song Y, Zhang LS. Immunotoxicity of atrazine in Balb/c mice[J]. J Environ Sci Health B, 2013, 48(8): 637–645. DOI:10.1080/03601234.2013.777308 |
| [16] | Zhao SH, Liu J, Zhao F, et al. Sub-acute exposure to the herbicide atrazine suppresses cell immune functions in adolescent mice[J]. Bioscience Trends, 2013, 7(4): 193–201. |
| [17] | Karrow NA, Mccay JA, Brown RD, et al. Oral exposure to atrazine modulates cell-mediated immune function and decreases host resistance to the B16F10 tumor model in female B6C3F1 mice[J]. Toxicology, 2005, 209(1): 15–28. DOI:10.1016/j.tox.2004.12.002 |
| [18] | Hooghe RJ. NTP report on the immunotoxicity of atrazine in female B6C3F1 mice (IMM94002)[J]. NTP, 1994, 1912(1): 24–29. |
| [19] | Pinchuk LM, Lee SR, Filipov LM, et al. In vitro atrazine exposure affects the phenotypic and function almaturation of dendritic cells[J]. Toxicol Applied Pharmacol, 2007, 223(3): 206–217. DOI:10.1016/j.taap.2007.06.004 |
| [20] | Karrow NA, McCay JA, Brown RD, et al. Oral exposure to atrazine modulates cell-mediated immune function and decreases host resistance to the B16F10 tumor model infemale B6C3F1 mice[J]. Toxicoligy, 2005, 209(1): 15–28. DOI:10.1016/j.tox.2004.12.002 |
| [21] | Ruiz VE, Sachdev M, Zhang SH, et al. Isolating, immunophenotyping and ex vivo stimulation of CD4(+) and CD8(+) gastric lymphocytes during murine Helicobacter pylori infection[J]. J Immunolog Methods, 2012, 384(1/2): 157–163. |
| [22] | Lee EJ, Jang Y, Kang K, et al. Atrazine induces endoplasmic reticulum stress-mediated apoptosis of T lymphocytes via the Caspase-8-dependent pathway[J]. Environmrnt Toxicol, 2016, 31(8): 998–1008. DOI:10.1002/tox.v31.8 |
| [23] | Wang X, Xing HJ, Li XL, et al. Effects of atrazine and chlorpyrifos on the mRNA levels of IL-1 and IFN-gamma 2b in immune organs of common carp[J]. Fish Shellfish Immunol, 2011, 31(1): 126–133. DOI:10.1016/j.fsi.2011.04.015 |
| [24] | Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer[J]. Cancer Treat Rev, 2012, 38(7): 904–910. DOI:10.1016/j.ctrv.2012.04.007 |
 2018, Vol. 44
2018, Vol. 44


