扩展功能
文章信息
- 潘鸿, 王宇峰, 黄海鹏, 张丽颖, 钟祯, 马诗棋, 董理, 王洪峰
- PAN Hong, WANG Yufeng, HUANG Haipeng, ZHANG Liying, ZHONG Zhen, MA Shiqi, DONG Li, WANG Hongfeng
- “调脏通络”电针对糖尿病周围神经病变大鼠坐骨神经细胞中GRP78表达的影响及其神经保护作用
- Influence of Adjust Zang Dredge Meridian electroacupuncture in expression of GRP78 in sciatic nerve cells of rats with diabetic peripheral neuropathy and its neuroprotective effect
- 吉林大学学报(医学版), 2018, 44(06): 1124-1130
- Journal of Jilin University (Medicine Edition), 2018, 44(06): 1124-1130
- 10.13481/j.1671-587x.20180603
-
文章历史
- 收稿日期: 2018-03-31
2. 北京市第一中西医结合医院儿科, 北京 100018;
3. 长春中医药大学针灸研究所, 吉林 长春 130117
2. Department of Pediatrics, Fist Hospital of Integrated Chinese and Western Medicine, Beijing City, Beijing 100018, China;
3. Institute of Acupuncture, Changchun University of Chinese Medicine, Changchun 130117, China
糖尿病周围神经病(diabetic peripheral neuropathy, DPN)是一种由糖尿病引起的以自主神经和感觉神经症状为主要临床表现的慢性并发症。现代医学研究[1]证明:在糖尿病的诸多并发症中,以DPN最为凶险。DPN发病机制尚不明确,高血糖、多元醇途径、氧化应激、炎性反应和神经生长因子等多种因素在DPN的发病过程中均起重要作用[2-6]。近年来,内质网应激(endoplasmic reticulum stress, ERS)作为一种新的细胞凋亡途径受到了广泛重视。研究[7]显示:ERS标志性蛋白坐骨神经葡萄糖调节蛋白78(glucose regulated protein 78,GRP78)在DPN大鼠坐骨神经中存在过度表达。目前西医治疗DPN主要采取控制血糖、改善微循环和营养神经等措施,其疗效不明显,且药物不良反应大[8]。相比之下,中医治疗尤其是电针疗法日益凸显其优势。中医治疗具有疗效确切、安全方便以及从整体、多途径、多靶点发挥镇痛及调节作用等优点。因此,本文作者采用“调脏通络”电针法干预糖尿病大鼠,检测其对GRP78表达的影响,探讨ERS介导的周围神经细胞凋亡在DPN发病中的作用,为临床治疗DPN提供依据。
1 材料与方法 1.1 实验动物SPF级成年雄性SD大鼠86只, 体质量180~220 g, 购于辽宁长生生物技术有限公司, 动物许可证编号:SCXK(辽)2015-0001。大鼠分笼饲料喂养,恒温21℃~25℃,湿度40%~70%,每日光照12 h,噪音<60 DB,喂养7 d后进行分组。
1.2 实验药物、主要试剂和仪器链脲佐菌素(STZ,美国Sigma公司),甲钴胺胶囊(江苏德源药业有限公司,规格:0.5 mg),GRP78抗体(英国Abcam公司),山羊血清(上海源叶生物科技有限公司),抗原修复液(北京鼎国昌盛生物技术有限责任公司),Hoechest33342 (英国Abcam公司),组织裂解液(美国Thermo公司)。BCA蛋白定量试剂盒(上海碧云天生物技术有限公司),酶标仪和湿电转膜仪(美国Bio Rad公司),垂直电泳槽(英国Abcam公司),ECL化学发光液试剂盒(上海天能科技有限公司),TUNEL试剂盒(英国Abcam公司)。
1.3 糖尿病大鼠模型制备将86只大鼠分为正常对照组(n=5)和造模组(n=81)。造模组大鼠适应性喂养1周,实验前检测空腹血糖值1次,所有大鼠空腹血糖值均小于5.8 mmol·L-1。造模组大鼠在造模前禁食(不禁水)12 h后称体质量,按50 mg·kg-1一次性腹腔注射1%STZ溶液,制备糖尿病模型。糖尿病模型成模标准为2次随机血糖值≥16.7 mmol·L-1,共79只大鼠造模成功。于糖尿病成模时(干预前)及干预12周后测定大鼠胫神经传导速度。
1.4 实验动物分组和干预糖尿病大鼠造模成功后,将79只成模大鼠随机分为模型对照组(n=27)、药物干预组(n=26)和电针干预组(n=26)。模型对照组:造模成功后,同等条件下饲养,不采取任何特殊处置,仅作对照观察。药物干预组:采取甲钴胺灌胃,20 mg·kg-1·d-1,每日1次,连续干预12周。电针干预组:取双侧肺俞、脾俞、肾俞、合谷、足三里、三阴交和太冲,予以“调脏通络”电针进行干预,选用华佗牌SDZ-V型电针仪,以同侧的肺俞-肾俞、三阴交-太冲为电针连接对穴,取疏密波(频率比为1:5),设置疏波频率为3 Hz,强度以刺激部位肌肉轻微收缩且大鼠能够耐受为度, 每日1次,每次20 min,6次为1个疗程,疗程间休息1 d,连续干预12周。腧穴定位参照国家十二五规划教材《实验针灸学》[9]中大鼠针灸穴位图谱进行定位,具体位置:肺俞,第3胸椎下两旁肋间;脾俞,第12胸椎下两旁肋间;肾俞,第2腰椎下两旁;合谷,前肢第1和第2掌骨之间;太冲,第1和第2跖骨间;足三里,膝关节后外侧,在腓骨小头下约5 mm处,在胫、腓骨间隙中;三阴交,后肢内踝尖直上10 mm。肺俞、脾俞、肾俞采用0.5寸毫针,直刺6 mm;合谷采用0.5寸毫针,直刺1 mm;太冲采用0.5寸毫针,直刺1 mm;足三里(后三里)采用0.5寸毫针,直刺7 mm;三阴交采用0.5寸毫针,直刺5 mm。正常对照组为“1.3”中未经造模、同等条件下饲养的大鼠。
1.5 大鼠热敏感度值的测定将大鼠放入52 ℃的RCY-2热板测痛仪(河北医科大学仪器厂)中, 从大鼠双后足接触热板到出现抬后足、突然摇晃身体或者舔足的时间作为热敏感度值指标。每次重复测定3次, 时间间隔5 min。于造模前、成模时及干预4和12周后测定大鼠平均热敏感度值。
1.6 大鼠胫神经传导速度测定各组大鼠给予水合氯醛腹腔注射麻醉,使用神经肌电图诱发电位仪, 在左侧坐骨神经切迹处采用双极针电极刺激左侧坐骨神经和胫神经远端至膝部,在腓肠肌部连接接收电极,尾骶部连接接地电极;在距离接收电极约0.5 mm处连接参考电极,脉冲12.8 mA、0.1 ms。测量刺激电极到接收电极的距离,测定胫神经的运动神经传导速度(motor nerve conduction velocity, MCV)和感觉神经传导速度(sensory nerve conduction velocity, SCV)。于成模时(干预前)及干预12周后各测定1次。
1.7 透射电镜观察各组大鼠坐骨神经超微结构针刺干预后,取大鼠坐骨神经于2.5%戊二醛溶液中固定过夜,磷酸缓冲液漂洗样品3次,采用1%锇酸固定样品1~2 h,梯度乙醇脱水,采用丙酮处理20 min,Epon812包埋剂梯度渗透,将样品置于Eppendorf管中包埋,于70℃过夜,包埋块呈黄色透明状,采用LKB-Ⅲ型超薄切片机半薄切片定位,经醋酸双氧铀及柠檬酸铅双重染色后于JEM-1200EX型透射电子显微镜下观察、拍片。
1.8 TUNEL法检测各组大鼠坐骨神经细胞凋亡率常规方法进行石蜡切片脱蜡,PBS洗涤3 min;快速将稀释的蛋白酶K滴加在组织上后采用PBS洗涤5 min;30%H2O2与甲醇按1:10的比例稀释成3%H2O2,覆盖组织后采用PBS冲洗5 min;采用TdT Equilibration和TdT Enzyme(试剂盒提供)进行平衡对消、标记反应;加入DAB工作液进行显色后进行苏木精染核,脱水、封片后,在荧光显微镜下观察各组细胞凋亡情况,计算细胞凋亡率。细胞凋亡率=凋亡细胞数/细胞总数×100%。
1.9 免疫荧光法检测各组大鼠坐骨神经中GRP78表达将坐骨神经石蜡切片脱蜡,依次浸入二甲苯、无水酒精、酒精、蒸馏水和PBS缓冲液中。将切片置于0.1 mol·L-1、pH=6.0的柠檬酸抗原修复液中,微波后取出,自然冷却至室温,采用PBS洗涤。采用山羊血清室温封闭孵育40 min。滴加一抗(1:200稀释),滴加荧光二抗(1:400稀释),取片,Hoechest染核,甘油封片,激光共聚焦显微镜下观察各组大鼠坐骨神经中GRP78的表达情况。
1.10 Western blotting法检测各组大鼠坐骨神经中GRP78表达水平称取约50 mg大鼠坐骨神经组织样本,加入200 μL组织裂解液,涡旋振荡器震荡,冰上裂解,4℃、14 000 g离心5 min, 取上清液,采用BCA法(二辛可酸法)进行蛋白定量。配制SDS-PAGE,加样电泳、转膜。采用TBST室温洗膜后,一抗孵育(GRP78、Actin与TBST按1:1 000稀释),二抗孵育(与5%脱脂奶粉按1:2 000稀释)。TBST洗膜,取出后加入ECL化学发光液(试剂盒提供),置于显影仪中曝光1 min显影。
1.11 统计学分析采用SPSS 22.0统计软件进行统计学分析。各组大鼠热敏感度值、胫神经的MCV和SCV、细胞凋亡率及坐骨神经中GRP78表达水平以x±s表示,组间比较采用单因素方差分析,组内比较采用t检验。以α=0.05为检验水准。
2 结果 2.1 各组大鼠热敏感度值糖尿病模型制备成功后,随着病程延长,大鼠热敏感度值逐渐降低。造模前和成模时,各组大鼠热敏感度值比较差异均无统计学意义(P>0.05);干预4和12周后,与模型对照组比较,药物干预组和电针干预组大鼠热敏感度值均明显升高(P<0.01);干预4周后,药物干预组和电针干预组大鼠热敏感度值比较差异无统计学意义(P>0.05);干预12周后,与药物干预组比较,电针干预组大鼠热敏感度值明显升高(P<0.01)。造模前与成模时各组大鼠组内热敏感度值比较差异均无统计学意义(P>0.05);干预4和12周后,模型组、药物干预组和电针干预组大鼠热敏感度值较造模前及成模时均明显降低(P<0.05)。见表 1。
| (x±s, t/s) | |||||
| Group | n | Thermal sensitivity value | |||
| Before modeling | Modeling | 4 weeks after intervention | 12 weeks after intervention | ||
| Normal control | 5 | 7.09±0.47 | 7.13±0.67 | 7.22±0.55 | 7.49±0.48 |
| Model control | 27 | 7.26±0.84 | 6.13±0.55 | 4.06±0.86△ | 2.82±0.20△ |
| Medicine intervention | 26 | 7.39±0.80 | 6.12±0.45 | 5.19±1.01*#○ | 3.91±0.58*#○ |
| Electroacupuncture intervention | 26 | 7.20±0.63 | 6.19±0.65 | 5.02±0.57*#○ | 5.67±0.60*△#○ |
| * P<0.01 vs model control group; △ P<0.05 vs medicine intervention group; # P<0.05 vs before modeling; ○ P<0.05 vs modeling. | |||||
干预前各组大鼠胫神经MCV和SCV比较差异均无统计学意义(P>0.05)。干预12周后组间比较:与模型对照组比较,药物干预组和电针干预组大鼠胫神经MCV和SCV升高(P<0.01);与药物干预组比较,电针干预组大鼠胫神经MCV和SCV升高,但组间比较差异无统计学意义(P>0.05)。组内比较:与干预前比较,干预12周后各组大鼠胫神经MCV和SCV均明显降低(P<0.01)。见表 2。
| [x±s,V/(m·s-1)] | ||||||
| Group | n | MCV | SCV | |||
| Before intervention | 12 weeks after intervention | Before intervention | 12 weeks after intervention | |||
| Normal control | 5 | 50.67±10.71 | 50.86±11.04 | 51.26±8.93 | 48.32±12.01 | |
| Model control | 27 | 45.00±9.44 | 20.63±10.27 | 46.28±11.65 | 21.43±11.51 | |
| Medicine intervention | 26 | 43.90±8.56 | 20.67±11.38*△ | 47.11±9.95 | 26.73±10.77*△ | |
| Electroacupuncture intervention |
26 | 43.84±9.14 | 30.26±8.96*△ | 45.13±9.49 | 29.54±9.39*△ | |
| * P<0.01 vs model control group; △ P<0.01 vs before intervention. | ||||||
正常对照组大鼠坐骨神经可见粗细不等的有髓神经纤维,神经轴索内电子密度均匀,髓鞘结构完整包绕在轴突外, 髓鞘板层规则排列,髓鞘结构完整致密、均匀规则。模型对照组可见部分髓鞘板层疏松呈不规则的膜样团块, 多见较粗的有髓神经纤维受累严重。药物干预组和电针干预组大鼠坐骨神经上述改变均减轻,有些神经纤维仍可见局部的板层分离现象。见图 1。
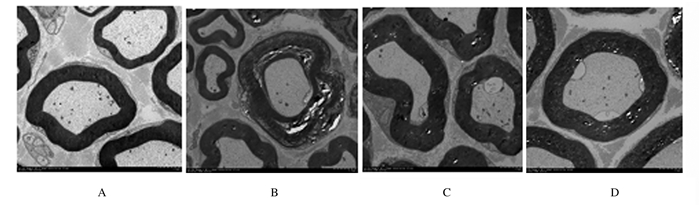
|
| A:Normal control group; B: Model control group; C:Medicine intervention group; D:Electroacupuncture intervention group. 图 1 透射电镜下各组大鼠坐骨神经超微结构(×1 500) Figure 1 Ultrastructures of sciatic nerves of rats in various groups under transmission electron microscope(×1 500) |
|
|
由于凋亡的细胞核内DNA断裂,暴露出3′端-OH,在末端脱氧核苷酸转移酶的作用下,加上HRP标记的dUTP,经过DAB后细胞呈现棕色,因此凋亡的细胞核呈现棕色,细胞核被苏木精染成蓝色。药物干预组和电针干预组大鼠TUNEL阳性细胞分布介于正常对照组及模型对照组之间;与正常对照组比较,模型对照组、药物干预组和电针干预组大鼠坐骨神经细胞凋亡率均明显升高(P<0.01);与模型对照组比较,药物干预组和电针干预组大鼠坐骨神经细胞凋亡率均降低(P<0.01), 药物干预组和电针干预组大鼠坐骨神经细胞凋亡率比较差异无统计学意义(P>0.05)。见图 2(插页一)和表 3。
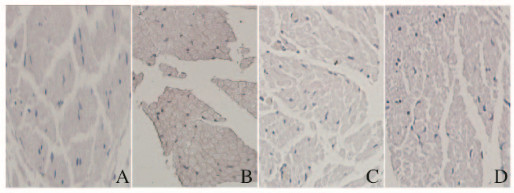
|
| A:Normal control group; B: Model control group; C:Electroacupuncture intervention group; D:Medicine intervention group. 图 2 各组大鼠坐骨神经细胞凋亡情况(TUNEL,×200) Figure 2 Apoptosis of sciatic nerve cells of rats in various groups(TUNEL, ×200) |
|
|
| (x±s) | |||
| Group | n | Apoptotic rate | GRP78 |
| Normal control | 5 | 0.084±0.022 | 0.21±0.05 |
| Model control | 27 | 0.342±0.130** | 0.48±0.18* |
| Medicine intervention | 26 | 0.129±0.013**△ | 0.32±0.10*△ |
| Electroacupuncture intervention | 26 | 0.138±0.020**△ | 0.29±0.07*△ |
| * P<0.05, * * P<0.01 vs normal control group;△ P<0.05 vs model control group. | |||
免疫荧光法检测可见:GRP78(红色)蛋白主要分布在神经髓鞘上。与正常对照组比较,模型对照组大鼠坐骨神经细胞中GRP78表达(即荧光强度)明显增强;与模型对照组比较,药物干预组和电针干预组大鼠坐骨神经细胞中GRP78表达(即荧光强度)明显减弱;药物干预组和电针干预组大鼠坐骨神经细胞中GRP78表达无明显差异。见图 3(插页一)。

|
| A:Normal control group; B: Model control group; C:Electroacupuncture intervention group; D:Medicine intervention group. 图 3 各组大鼠坐骨神经细胞中GRP78的表达(免疫荧光,×600) Figure 3 Expressions of GRP78 in sciatic nerve cells of rats in various groups (Immunofluorescence, ×600) |
|
|
与正常对照组比较,模型对照组、药物干预组和电针干预组大鼠坐骨神经中GRP78表达水平明显升高(P<0.05);与模型对照组比较,药物干预组和电针干预组大鼠坐骨神经细胞中GRP78表达水平明显降低(P<0.05);药物干预组和电针干预组大鼠坐骨神经细胞中GRP78表达水平比较差异无统计学意义(P>0.05)。见图 4和表 3。
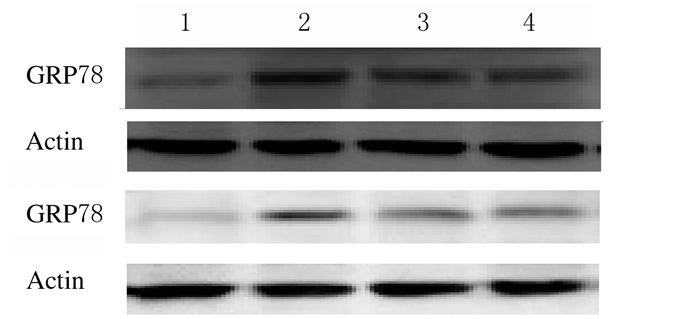
|
| Lane 1:Normal control group;Lane 2:Model control group;Lane 3:Electroacupuncture intervention group;Lane 4:Medicine intervention group. 图 4 各组大鼠坐骨神经中GRP78表达电泳图 Figure 4 Electrophoregram of GRP78 in sciatic nerve cells of rats in various groups |
|
|
DPN是糖尿病最常见的慢性并发症之一。该病具有起病隐匿和发生率高的特点,但其机制尚未完全阐明。因此,深入研究DPN发病机制及其保护效应对于日后临床预防和治疗DPN具有重要指导意义。研究[10]证实:STZ诱导的糖尿病大鼠造模成功后1~2个月可以发展成DPN,本文作者在糖尿病成模后即给予“调脏通络”电针干预,以观察“调脏通络”电针对DPN大鼠热敏感度值、胫神经MCV和SCV的影响。
GRP78属于热休克蛋白70(HSP70)家族成员, 是存在于内质网上最多的分子伴侣蛋白,ERS时通过未折叠蛋白反应可使GRP78大量表达, 被认为是ERS的标志性分子[11-12]。实验[13]表明:GRP78作为ERS的标志性蛋白在DPN大鼠坐骨神经细胞中存在过度表达,具有协助蛋白质折叠、维持内质网钙稳态和减轻ERS的作用。GRP78的C末端可与蛋白折叠中间产物的疏水区域结合, N末端具有ATP酶活性, 通过水解ATP的耗能过程防止蛋白质聚集并促进其折叠以获得正确的空间构象并维持内质网钙稳态, 以减轻ERS[14]。
本研究采用STZ诱导建立DPN大鼠模型,采用免疫荧光和Western blotting法进行检测,以肺俞、脾俞、肾俞、足三里、三阴交、合谷和太冲为主穴,行“调脏通络”电针刺激。本研究结果表明:在DPN疾病进程中,大鼠热敏感度值和坐骨神经远端胫神经传导速度明显降低,坐骨神经细胞中GRP78表达水平明显升高,经“调脏通络”电针治疗后坐骨神经细胞凋亡率和GRP78表达水平明显低于模型对照组,有髓神经纤维板层分离现象减轻, 提示“调脏通络”电针对神经凋亡保护的效应之一可能是通过减少GRP78表达,从而抑制ERS的发生,促进神经纤维再生,改善板层分离现象。但目前关于GRP78的治疗作用机制尚未明确。关于神经细胞、视网膜细胞和巨噬细胞等研究[15-17]显示:GRP78对细胞起保护作用, 延长各种不利因素刺激下的细胞生存期, 调节细胞凋亡。Ermakova等[18]发现:GRP78一方面通过细胞内蛋白折叠,靶向降解错误折叠蛋白发挥分子伴侣作用;另一方面,GRP78表达水平上调可抑制细胞凋亡, 可能与结合并抑制雌激素受体(ER)上的促凋亡受体有关,有助于细胞抵抗应激, 维持细胞存活。Ranganathan等[19]发现:细胞内给予p38MAPK特异性激动剂后,GRP78表达水平明显升高,提示P38MAPK途径可能参与了细胞表达调控过程,在细胞生长、应激和炎症抑制等方面发挥重要作用。本课题组前期实验结果表明:GRP78在ERS介导坐骨神经细胞凋亡中存在过度表达,在DPN发生发展过程中起重要调控作用。同时本研究选择“调脏通络”电针疗法作为治疗DPN主要思路,结合辩证选穴和近部选穴的选穴原则[20],形成电针最优治疗方案。本文作者认为:“调脏通络”电针可以改善胫神经传导速度及周围神经病理形态, 降低神经细胞凋亡率,减少GRP78表达,从而对坐骨神经起保护作用,说明“调脏通络”电针对DPN大鼠周围神经细胞凋亡的保护效应机制可能是通过抑制ERS而实现。
综上所述,“调脏通络”电针疗法可以减少DPN坐骨神经细胞中GRP78表达。本研究结果既为“调脏通络”电针的临床应用提供了高质量的实验证据,也为以ERS为靶点的DPN治疗提供了新的策略和科学依据。GRP78表达水平降低是通过何种途径、电针干预是否为GRP78表达水平降低的直接原因是未来研究的重点。
| [1] | Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies:a statement by the American Diabetes Association[J]. Diabetes Care, 2005, 28(4): 956–962. DOI:10.2337/diacare.28.4.956 |
| [2] | Hamid HS, Mervak CM, Münch AE, et al. Hyperglycemia-and neuropathy-induced changes in mitochondria within sensory nerves[J]. Ann Clin Transl Neurol, 2014, 1(10): 799–812. DOI:10.1002/acn3.119 |
| [3] | Edwards JL, Vincent AM, Cheng HT, et al. Diabetic neuropathy:mechanisms to management[J]. Pharmacol Ther, 2008, 120(1): 1–34. DOI:10.1016/j.pharmthera.2008.05.005 |
| [4] | Sandireddy R, Yerra VG, Areti A, et al. Neuroinflammation and oxidative stress in diabetic neuropathy:futuristic strategies based on these targets[J]. Int J Endocrinol, 2014, 2014(1): 674987. |
| [5] | Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome:Immune-inflammatory features as possible cardiovascular markers in diabetes[J]. WorldJ Orthop, 2015, 6(1): 62–76. |
| [6] | Lane JT. The role of retinoids in the induction of nerve growth factor:a potential treatment for diabetic neuropathy[J]. Transl Res, 2014, 164(3): 193–195. |
| [7] | Lupachyk S, Watcho P, Stavnllchuk R, et al. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy[J]. Diabetes, 2013, 62(3): 944–952. |
| [8] | 杨欢, 郑小兰, 徐国海, 等. 电针治疗糖尿病神经病理性疼痛机制的研究进展[J]. 北京:中国疼痛医学杂志, 2015, 21(11): 855–858. |
| [9] | 郭义, 方剑乔. 实验针灸学[M]. 北京: 中国中医药出版社,2012: 46-49. |
| [10] | Yangihashi S, Kamijo M, Watanabe K. Reduced mychanted fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats[J]. Am J Pathol, 1990, 136(6): 1363–1373. |
| [11] | Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration[J]. Cell Calcium, 2005, 38(3/4): 409–415. |
| [12] | Liu H, Qian J, Wang F. Expression of two endoplasmic reticulum stress markers, GRP78 and GADD153, in rats retinal detachment model and its implication[J]. Eye(Lond), 2010, 24(1): 137. |
| [13] | Lupachyk S, Watcho P, Stavniichuk R, et al. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy[J]. Diabetes, 2013, 62(3): 944–952. |
| [14] | Yang GH, Li S, Pestka JJ. Down-regulation of the endoplasmic reticulum chaperone GRP78/BiP by vomitoxin (Deoxynivalenol)[J]. Toxicol Appl Pharmacol, 2000, 162(3): 207–217. DOI:10.1006/taap.1999.8842 |
| [15] | Hayashi T, Saito A, Okuno S, et al. Induction of GRP78 by ischemic preconditioning reduces endoplasmic reticulum stress and prevents delayed neuronal cell death[J]. J Cereb Blood Flow Metab, 2003, 23(8): 949–961. DOI:10.1097/01.WCB.0000077641.41248.EA |
| [16] | Oida Y, Izuta H, Oyagi A, et al. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil[J]. Brain Res, 2008, 1208: 217–224. DOI:10.1016/j.brainres.2008.02.068 |
| [17] | Reddy RK, Mao C, Baumeister P, et al. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors:role of ATP binding site in suppression of caspase-7 activation[J]. Biol Chem, 2003, 278(23): 20915–20924. DOI:10.1074/jbc.M212328200 |
| [18] | Ermakova SP, Kang BS, Choi BY, et al. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78[J]. Cancer Res, 2006, 66(18): 9260–9269. DOI:10.1158/0008-5472.CAN-06-1586 |
| [19] | Ranganathan AC, Zhang L, Adam AP, et al. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells[J]. Cancer Res, 2006, 66(3): 1702–1711. DOI:10.1158/0008-5472.CAN-05-3092 |
| [20] | 陈晓蕾, 贾农, 柯亭羽, 等. 2型糖尿病合并亚临床甲状腺功能减低与肾脏损害相关分析[J]. 中国实用内科杂志, 2018, 38(1): 65–68. |
 2018, Vol. 44
2018, Vol. 44


