扩展功能
文章信息
- 于洋, 徐路, 刘师兵, 李松岩, 徐冶
- YU Yang, XU Lu, LIU Shibing, LI Songyan, XU Ye
- 杨梅素通过促进DRP1依赖性线粒体分裂诱导人卵巢癌SKOV3细胞凋亡
- Induction effect of myricetin on apoptosis of human ovarian cancer SKOV3 cells by promoting DRP1-dependent mitochondrial fission
- 吉林大学学报(医学版), 2018, 44(05): 903-907
- Journal of Jilin University (Medicine Edition), 2018, 44(05): 903-907
- 10.13481/j.1671-587x.20180503
-
文章历史
- 收稿日期: 2017-06-16
杨梅素是提取于杨梅科杨梅属植物杨梅果实的一种酮类化合物。植物黄酮类均具有较强的抗氧化活性[1-2],使其具有一定抑菌作用[3-4]、免疫因子激活作用[5-6]和抗衰老作用[7-8]等生物学活性;曾有研究者[9]对杨梅素的抑制肿瘤生长作用作出过相应报道,但具体机制尚不十分明确。线粒体功能的维持是细胞正常运转的重要保证,线粒体动力学是目前针对于线粒体功能研究的焦点之一,主要包括内外线粒体膜的融合和分裂,这个相反的过程有助于维持线粒体的正常生理功能[10-11]。线粒体过度融合常导致巨型线粒体的产生,常见于病变肝细胞或营养不良患者胰腺细胞等[12];反之,若线粒体过度分裂,则会导致线粒体形成碎片,使线粒体部分或完全丧失基本功能,诱发线粒体自噬甚至导致线粒体途径凋亡的发生[13]。卵巢癌发病率位居女性生殖肿瘤第3位,目前已成为女性生殖肿瘤致死的首要病因[14]。在临床化疗过程中出现的不同程度肿瘤耐药促使人们找寻更为低毒高效的生物制剂或化学药物来抑制或杀灭肿瘤细胞。本课题组前期研究[15]显示:杨梅素对于人卵巢癌细胞有明显的凋亡诱导作用。本研究在前期研究基础上,以杨梅素作用于人卵巢癌SKOV3细胞,结合特异性动力相关蛋白1(dynamin related protein 1, DRP1)抑制剂—mdivi/1,观察杨梅素诱导SKOV3细胞株凋亡过程中线粒体分裂情况,探讨杨梅素抗肿瘤的作用机制。
1 材料与方法 1.1 细胞、主要试剂和仪器SKOV3细胞由吉林大学病理生理学系惠赠。Muse®细胞状态分析仪凋亡检测试剂盒购于美国Muse®试剂公司,所有一抗均购于美国Santa Cruz公司,新生牛血清、RPMI1640培养基购于美国Gibco®公司,二抗及其他试剂购于北京鼎国生物试剂公司。860型酶标仪,美国Bio-Rad公司;化学发光仪,中国上海天能科技有限公司;FV1000型共聚焦显微镜,日本Olympus公司;Muse®细胞状态分析仪,美国默克密理博公司。
1.2 细胞培养和分组SKOV3细胞用含体积分数为10%新生牛血清的1640培养液,置37℃、5% CO2培养箱中培养,隔2~3 d传代1次。取生长状态良好对数生长期细胞,随机分为对照组、mdivi-1组、杨梅素组和联合给药组,对照组不做干预,mdivi-1组以50 μmol·L-1 mdivi-1作用1 h后更换含细胞培养液继续培养23 h,杨梅素组以50 g·L-1杨梅素作用24 h,联合给药组以50 μmol·L-1 mdivi-1作用1 h后更换含50 g·L-1杨梅素的细胞培养液继续培养23 h。
1.3 MTT法检测各组细胞存活率先将细胞接种至96孔板,按1.2中分组方法进行分组及给药处理24 h后,每孔加入5g·L-1 MTT溶液10 μL,继续培养4 h。弃上清,每孔加入100μL二甲基亚砜,置摇床上低速振荡10 min,使结晶物充分溶解。最后以酶标仪测490 nm处各孔吸光度(A)值,计算细胞存活率。细胞存活率=(给药组A值-空白组A值)/(对照组A值-空白组A值)×100%。
1.4 细胞凋亡率的检测严格按照Muse®细胞状态分析仪凋亡检测试剂盒说明书进行操作,并利用Muse®细胞状态分析仪进行检测。细胞凋亡率=早期凋亡率+晚期凋亡率。每组细胞平行检测6个样品,最终结果以x±s表示。
1.5 Western blotting法检测各组细胞中相关蛋白表达水平按1.2中分组方法分组并干预SKOV3细胞,以胰蛋白酶消化离心收集全部细胞,收集后的每管细胞加入120 μL RIPA细胞裂解液,混匀后,冰浴下用细胞超声粉碎仪超声粉碎2次,每次5~10 s,放入4℃冰箱45 min,充分裂解细胞。离心,收集上清液,以96孔板蛋白定量后,所余部分上清液与5×SDS Loading缓冲液按4︰1体积比混匀后进行蛋白变性。变性后的蛋白提取液进行SDS-PAGE电泳,电泳条件:浓缩胶100 V、30 min、分离胶200 V、60 min,电泳结束后,利用湿转法将胶内蛋白转移到PVDF膜上,将膜用5%脱脂奶粉封闭1.5 h后,PBST洗3次,加入以一抗稀释液200倍稀释后的检测目标一抗,分别为:β-肌动蛋白(β-actin)、细胞色素C(cytochrome C,Cyt C)、半胱氨酸天冬氨酸蛋白酶3(cysteine-containing aspartate-specific proteases 3,caspase3)、动力相关蛋白1(dynamin related protein 1,DRP1)和分裂蛋白1(fission 1,FIS1),4℃孵育过夜。次日PBST洗3次,加入过氧化物酶标记的相应二抗(1︰1 000稀释),室温下摇床摇1 h,PBST洗3次,ECL发光并拍照,以β-actin作为内参照对各组蛋白表达变化灰度进行对比分析,并对蛋白表达水平进行统计学分析。蛋白表达水平=每个样本条带灰度值/β-actin灰度值。
1.6 MitoTracker® Red观察线粒体形态按1.2中分组方法进行分组及给药处理,制备细胞爬片后,以MitoTracker® Red 250 μmol·L-1,37℃孵育30 min;以Hoechst 33342染核2 min后,激光共聚焦显微镜观察线粒体形态。
1.7 统计学分析采用SPSS 13.0软件进行统计学分析。各组SKOV3细胞存活率,细胞凋亡率和Cyt C、caspase 3、DRP1和FIS1蛋白表达水平以x±s表示,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组人卵巢癌SKOV3细胞存活率与对照组(100%)比较,mdivi-1组细胞存活率(97.28%± 4.12%)无明显降低(P>0.05),杨梅素组细胞存活率(52.17%±6.28%)明显降低(P < 0.05),而联合给药组细胞存活率(63.87%±5.68%)明显高于杨梅素组(P < 0.05)。
2.2 各组人卵巢癌SKOV3细胞凋亡率与对照组[细胞凋亡率(5.89%±1.12%)]比较,mdivi-1组细胞凋亡率(5.99%±2.28%)无明显变化(P>0.05),杨梅素组细胞凋亡率(45.35%± 5.38%)升高(P < 0.05),联合用药组细胞凋亡率(32.62%±4.47%)低于杨梅素组(P < 0.05)。见图 1。

|
| A:Control group; B:Mdivi-1 group; C:Myricetin group; D:Combined group. 图 1 Muse®检测各组SKOV3细胞凋亡率 Figure 1 Apoptotic rates of SKOV3 cells in various groups detected by Muse® |
|
|
与对照组比较,杨梅素组细胞中Cyt C和caspase3蛋白表达水平明显升高(P < 0.05);联合用药组细胞中Cyt C和caspase3蛋白表达水平较杨梅素组均明显下降(P < 0.05)。见图 2。
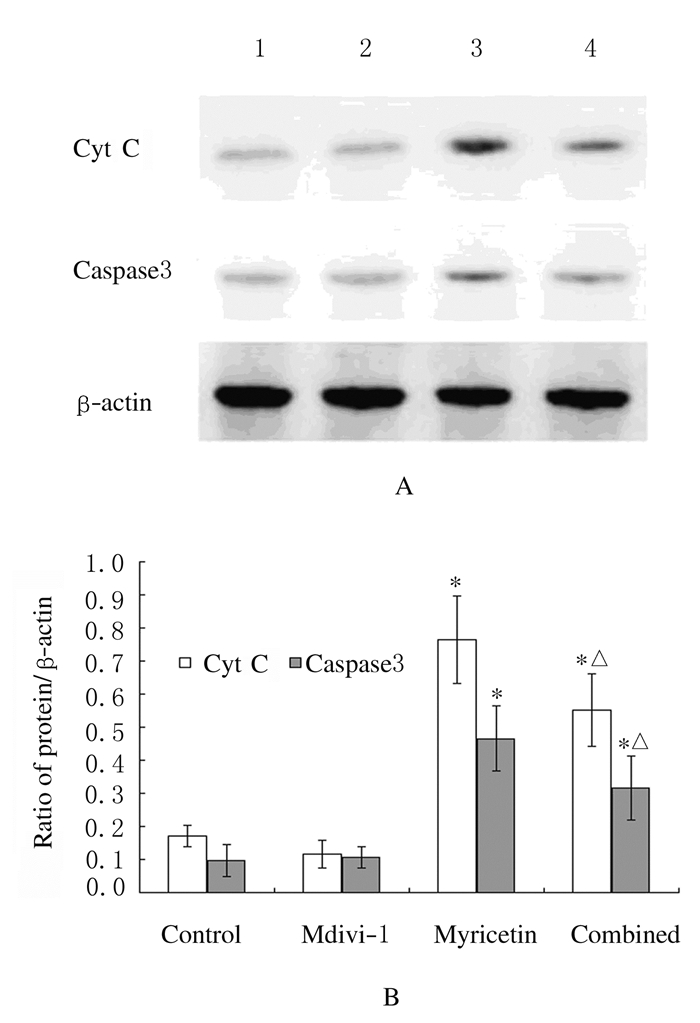
|
| Lane 1:Control group; Lane 2:Mdivi-1 group; Lane 3:Myricetin group; Lane 4:Combined group. *P < 0.05 vs control group; △P < 0.05 vs myricetin group. 图 2 各组SKOV3细胞中Cyt C和caspase3蛋白表达电泳图(A)和直条图(B) Figure 2 Electrophorogram(A) and histogram(B) of expressions of Cyt C and caspase3 proteins in SKOV3 cells in various groups |
|
|
经MitoTracker® Red荧光探针特异性标记线粒体后激光共聚焦显微镜观察线粒体分裂情况,结果显示:mdivi-1组细胞中线粒体荧光染色表现与对照组基本一致;杨梅素组细胞内红色荧光增强,且较对照组线粒体的条索样结构呈现明显粗糙砂砾样改变,说明杨梅素组细胞内线粒体分裂趋势较对照组增强;与杨梅素组比较,联合给药组细胞中线粒体荧光染色砂砾感下降,亮度减弱,说明线粒体分裂程度下降。见图 3(插页一)。
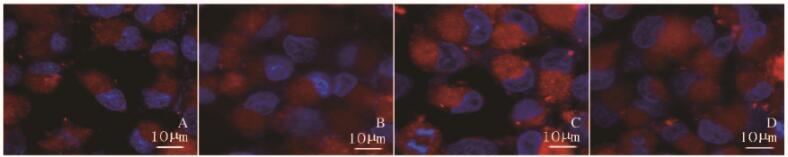
|
| 图 3 各组SKOV3细胞线粒体形态表现(Bar=10 μm) Figure 3 Morphology of mitochondria of SKOV3 cells in various groups(Bar=10 μm) |
|
|
Western blotting结果显示:杨梅素组细胞中DRP1和FIS1表达水平较对照组明显升高(P < 0.05),而联合用药组DRP1和FIS1表达水平较杨梅素组均明显降低(P < 0.05)。见图 4。
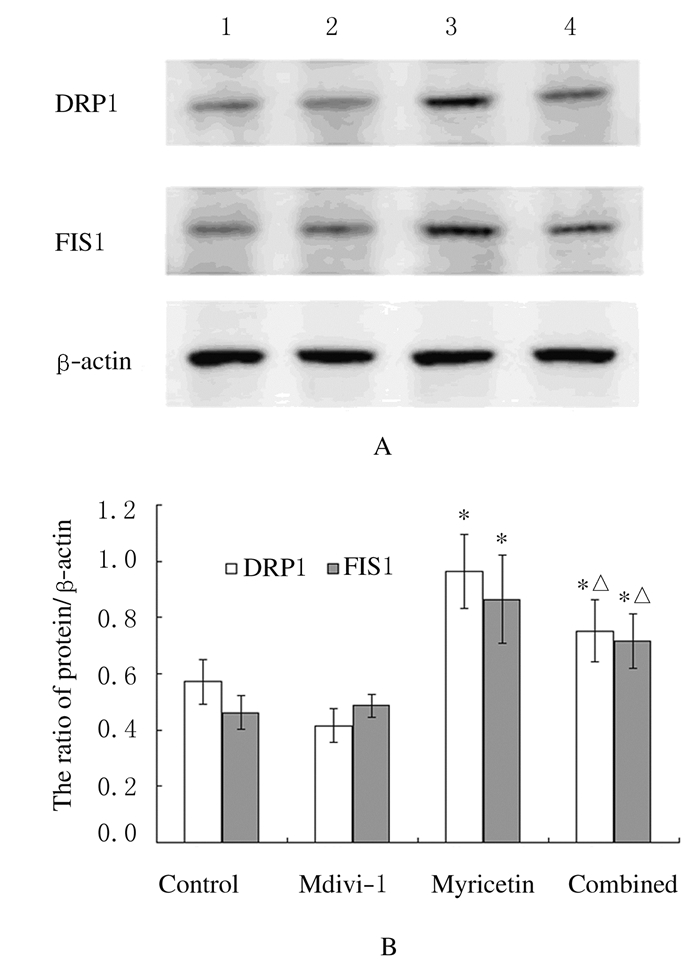
|
| Lane 1:Control group; Lane 2:Mdivi-1 group; Lane 3:Myricetin group; Lane 4:Combined group. *P < 0.05 vs control group; △P < 0.05 vs myricetin group. 图 4 各组SKOV3细胞中DRP1和FIS1蛋白表达电泳图(A)和直条图(B) Figure 4 Electrophorogram(A) and histogram(B) of expressions of DRP1 and FIS1 proteins in SKOV3 cells in various groups |
|
|
线粒体是真核细胞内的重要细胞器,其参与能量代谢、信号传导和细胞凋亡等众多生理病理学过程。线粒体具有网状结构,且由于线粒体的分裂和融合,致使该网状结构长期处于动态变化中。新近研究[16]证实:线粒体分裂是细胞凋亡过程早期发生的细胞器形态改变,而线粒体融合则对肿瘤细胞凋亡有抑制作用[17]。线粒体分裂和融合过程受多种蛋白的精确调控,线粒体分裂对外界刺激和代谢信号高度敏感。研究[18-19]表明:多种信号通路和大量信号分子参与该过程。DRP1是调节线粒体融合和分裂的关键蛋白,正常情况下其主要定位于细胞质,经过裂变信号刺激,DRP1转移到线粒体,与FIS1结合共同参与线粒体分裂过程[13]。本研究将杨梅素作用于人卵巢癌SKOV3细胞,结果显示:杨梅素具有明显降低细胞存活率和凋亡诱导作用,Cyt C和caspase3表达水平变化提示该凋亡与线粒体功能有密切关联,线粒体荧光探针和DRP1、FIS1蛋白表达水平变化也提示杨梅素组SKOV3细胞内线粒体分裂明显增强。为了进一步明确杨梅素所诱导凋亡的具体机制及与线粒体分裂之间的关系,本研究进一步引入mdivi-1,通过抑制DRP1功能抑制线粒体分裂[20-22],将其与杨梅素联用后,杨梅素的凋亡诱导作用明显受到抑制,说明杨梅素该作用是DRP1依赖性的,杨梅素很可能通过上调DRP1表达来促进SKOV3细胞线粒体分裂从而发挥凋亡诱导作用。本研究结果在相关杨梅素抗癌作用的研究中是较为新颖的发现,国内外尚无相关类似报道。本研究结果对完善杨梅素及其同类衍生物抗癌作用的机制具有一定意义,也为新型低毒高效抗肿瘤药物的研发提供参考。
| [1] | Xiao S, Mu ZQ, Cheng CR, et al. Three new biflavonoids from the branches and leaves of Cephalotaxus oliveri and their antioxidant activity[J]. Nat Prod Res, 2018, 15: 1–7. |
| [2] | Tian Y, Puganen A, Alakomi HL, et al. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants[J]. Food Res Int, 2018, 106: 291–303. DOI:10.1016/j.foodres.2017.12.071 |
| [3] | Sharma K, Mahato N, Lee YR. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions[J]. J Food Drug Anal, 2018, 26(2): 518–528. DOI:10.1016/j.jfda.2017.06.009 |
| [4] | Khalfallah A, Berrehal D, Bensouici C, et al. Flavonoids, cytotoxic, antioxidant and antibacterial activities of Evax pygmaea[J]. Pharm Biol, 2017, 55(1): 2292–2296. DOI:10.1080/13880209.2017.1405997 |
| [5] | Hariri BM, McMahon DB, Chen B, et al. Plant flavones enhance antimicrobial activity of respiratory epithelial cell secretions against Pseudomonas aeruginosa[J]. PLoS One, 2017, 12(9): e0185203. DOI:10.1371/journal.pone.0185203 |
| [6] | Taverniti V, Dalla Via A, Minuzzo M, et al. In vitro assessment of the ability of probiotics, blueberry and food carbohydrates to prevent S. pyogenes adhesion on pharyngeal epithelium and modulate immune responses[J]. Food Funct, 2017, 8(10): 3601–3609. DOI:10.1039/C7FO00829E |
| [7] | Choi S, Youn J, Kim K, et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo[J]. Int J Mol Med, 2016, 38(2): 627–634. DOI:10.3892/ijmm.2016.2626 |
| [8] | Wei J, Zhang G, Zhang X, et al. Anthocyanins from black chokeberry (aroniamelanocarpa elliot) delayed aging-related degenerative changes of brain[J]. J Agric Food Chem, 2017, 65(29): 5973–5984. DOI:10.1021/acs.jafc.7b02136 |
| [9] | Martínez-Pérez C, Ward C, Turnbull AK, et al. Antitumour activity of the novel flavonoid Oncamex in preclinical breast cancer models[J]. Br J Cancer, 2016, 114(8): 905–916. DOI:10.1038/bjc.2016.6 |
| [10] | Autret A, Martin SJ. Bcl-2 family proteins and mitochondrial fission/fusion dynamics[J]. Cell Mol Life Sci, 2010, 67(10): 1599–1606. DOI:10.1007/s00018-010-0286-x |
| [11] | Meyer JN, Hartman JH, Mello DF. Mitochondrial Toxicity[J]. Toxicol Sci, 2018, 162(1): 15–23. DOI:10.1093/toxsci/kfy008 |
| [12] | Cabrera-Serrano M, Junckerstorff RC, Atkinson V, et al. Novel CHKB mutation expands the megaconial muscular dystrophy phenotype[J]. Muscle Nerve, 2015, 51(1): 140–143. DOI:10.1002/mus.v51.1 |
| [13] | Zhao C, Chen Z, Qi J, et al. Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function[J]. Oncotarget, 2017, 8(13): 20988–21000. |
| [14] | Manchanda R, Legood R, Antoniou AC, et al. Changing the risk threshold for surgical prevention of ovarian cancer[J]. BJOG, 2018, 125(5): 541–544. DOI:10.1111/bjo.2018.125.issue-5 |
| [15] | 于洋, 刘亚萌, 吴少花, 等. 杨梅素对人卵巢癌SKOV3细胞的凋亡诱导作用[J]. 吉林大学学报:医学版, 2015, 41(5): 902–906. |
| [16] | Sehrawat A, Croix CS, Baty CJ, et al. Inhibition of mitochondrial fusion is an early and critical event in breast cancer cell apoptosis by dietary chemopreventative benzyl isothiocyanate[J]. Mitochondrion, 2016, 30: 67–77. DOI:10.1016/j.mito.2016.06.006 |
| [17] | Chen SD, Zhen YY, Lin JW, et al. Dynamin-related protein 1 promotes mitochondrial fission and contributes to the hippocampal neuronal cell death following experimental status epilepticus[J]. CNS Neurosci Ther, 2016, 22(12): 988–999. DOI:10.1111/cns.2016.22.issue-12 |
| [18] | Huang G, Massoudi D, Muir AM, et al. WBSCR16 is a guanine nucleotide exchange factor important for mitochondrial fusion[J]. Cell Rep, 2017, 20(4): 923–934. DOI:10.1016/j.celrep.2017.06.090 |
| [19] | Fan LF, He PY, Peng YC, et al. Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis[J]. Free Radic Biol Med, 2017, 112: 336–349. DOI:10.1016/j.freeradbiomed.2017.08.003 |
| [20] | Hu C, Huang Y, Li L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals[J]. Int J Mol Sci, 2017, 18(1): E144. DOI:10.3390/ijms18010144 |
| [21] | Li GB, Fu RQ, Shen HM, et al. Polyphyllin I induces mitophagic and apoptotic cell death in human breast cancer cells by increasing mitochondrial PINK1 levels[J]. Oncotarget, 2017, 8(6): 10359–10374. |
| [22] | Han XJ, Yang ZJ, Jiang LP, et al. Mitochondrial dynamics regulates hypoxia-induced migration and antineoplastic activity of cisplatin in breast cancer cells[J]. Int J Oncol, 2015, 46(2): 691–700. DOI:10.3892/ijo.2014.2781 |
 2018, Vol. 44
2018, Vol. 44


