扩展功能
文章信息
- 白洁, 纪文静, 丁永年, 彭媛媛, 陈源文
- BAI Jie, JI Wenjing, DING Yongnian, PENG Yuanyuan, CHEN Yuanwen
- 胆总管结扎致肝纤维化模型大鼠肝脏内源性AcSDKP及其调控因子水平的变化
- Changes of levelsof endogenous AcSDKP and its regulatory factors in liver tissue of model rats with liver fibrosis induced by bile duct ligation
- 吉林大学学报(医学版), 2018, 44(05): 999-1004
- Journal of Jilin University (Medicine Edition), 2018, 44(05): 999-1004
- 10.13481/j.1671-587x.20180520
-
文章历史
- 收稿日期: 2017-11-21
2. 上海交通大学医学院附属新华医院消化内科, 上海 200092
2. Department of Gastroenterology, Xinhua Hospital, School of Medical Sciences, Shanghai Jiaotong University, Shanghai 200092, China
N-乙酰基-丝氨酰-天门冬酰-赖氨酰-脯氨酸(N-acetyl-seryl-aspartyl-lysylproline, AcSDKP)是1种内源性乙酰化四肽化合物,其前体胸腺素β4通过脯氨酸寡肽酶(prolyl oligopeptidase,POP)水解生成,胸腺素β4-POP-AcSDKP轴广泛存于哺乳动物多种器官与体液中[1]。研究[2]证实:AcSDKP具有多种重要生物学功能,其中对心脏、肾脏等重要器官的纤维化或重塑具有较强的抑制作用。近年研究[3]显示:肝纤维化发生发展过程中AcSDKP起着极为重要的效应,在肝细胞外基质沉积以及肝损伤修复等方面具有重要调控作用。目前研究[4]证实:血管紧张素转换酶(angiotensin converting enzyme,ACE)是内源性AcSDKP特异性水解酶,在体内外均有极强的AcSDKP水解活性。目前这些调控作用的具体机制尚不清楚,因此也是临床研究的热点问题之一。肝纤维化动物模型较多,其中胆总管结扎肝纤维化模型是应用较为普遍的方法之一,因此本研究选择胆总管结扎制备大鼠肝纤维化模型,分析肝纤维化模型大鼠内源性AcSDKP的动态变化以及其调节因素的改变情况,以期为临床治疗肝纤维化提供基础资料。
1 材料与方法 1.1 实验动物、主要试剂和仪器普通级4周龄健康雄性Sprague-Dawley(SD)大鼠45只,购自北京维通利华实验动物技术有限公司,体质量(190±18)g,动物合格证号:SCXK(京)2012-0001;均予以动物食用标准饲料,维系12 h光照的昼夜周期,且本研究对动物处置方法符合上海交通大学医学院附属新华医院伦理委员会要求。Ⅲ型前胶原(PCⅢ)、Ⅳ型胶原(CⅣ)和透明质酸(HA)试剂盒均购自上海信裕生物技术有限公司,AcSDKP检测ELISA试剂盒购自法国BIO公司。RM2255型号石蜡切片机购自德国莱卡公司,Multi-analyst Software凝胶图像分析处理软件购自美国Gel公司,AU5800型自动生化分析仪购自美国贝克曼公司。
1.2 实验动物分组按照随机数字表法将45只SD大鼠分为3组,每组15只:模型组,采用胆总管结扎建立肝纤维化动物模型;阻断组,大鼠予以血管紧张素转化酶抑制剂(ACEI)卡托普利(40 mg·kg-1)每天灌胃1次,连续灌胃8周,再采用胆总管结扎建立肝纤维化动物模型;对照组,予以开腹,但不结扎胆总管。3组大鼠的周龄、体质量、饮食以及大小便等因素比较差异均无统计学意义(P>0.05),具有可比性。
1.3 肝纤维化动物模型的建立SD大鼠正常适应性喂养1周后,自尾静脉内注射苯巴比妥2 mg·kg-1,消毒后开腹,分离胆总管并予以结扎,再逐步关腹,术后予以常规喂养,本研究中所有大鼠肝纤维化实验模型均通过肝脏病理检查证实造模建立成功[5]。3组大鼠均分别在1、2和4周后各处死5只动物。
1.4 肝功能和纤维化指标检测3组大鼠均在处死前经下腔静脉采集静脉血,以3 000 r·min-1离心后,收集上层血清标本,保存在-20℃冰箱中待检查。应用自动生化分析仪检测血清样本中丙氨酸氨基转移酶(ALT)、门冬氨酸氨基转移酶(AST)、总胆红素(TB)和白蛋白(ALB)水平。应用放射免疫法测定血清样本中PCⅢ、CⅣ和HA水平。
1.5 肝纤维化组织病理学观察肝组织标本分为2份,一份肝组织置于液氮冷冻后,-80℃冰箱中保存,采用ELISA法检测肝脏组织样本中AcSDKP水平;另一份肝组织置于10%缓冲甲醛中固定24 h,石蜡包埋,组织病理切片层厚4 μm,HE染色,评估肝脏肝组织纤维化及重构情况。
1.6 胸腺素β4、POP和ACE蛋白表达水平检测应用Western blotting法半定量分析检测3组大鼠肝组织中胸腺素β4、POP和ACE蛋白表达水平,并比较1、2和4周时间点上述3种指标动态变化。实验具体步骤:取0.5 g冰冻肝组织置于4℃的组织匀浆缓冲液中匀浆,12 000 r·min-1高速离心15 min,取上清液,样品的蛋白浓度按Bradford法以牛血清白蛋白(BSA)为标准测定,根据标准曲线推算出样品的蛋白浓度。然后取100 μg总蛋白沸水浴10 min后进行10%SDS-PAGE电泳,以β-actin为内参。电泳结束后进行Western blotting法检测,应用Gel公司的Multi-analyst Software软件进行吸光度(A)值定量分析,结果以目的蛋白条带与内参蛋白条带A值的比值表示,以确定胸腺素β4、POP和ACE蛋白表达水平。
1.7 统计学分析采用SPSS 17.0软件进行统计学分析。肝功能指标(ALT、AST和TB等)、肝纤维化指标(PCⅢ、CⅣ和HA等)和肝组织AcSDKP水平为计量资料,先予以正态分布检验和方差齐性检验,均为正态分布及方差齐性,以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用SNK-q检验。胸腺素β4、POP和ACE表达水平为计数资料,以倍数的形式表示,各组间倍数的比较采用χ2检验。以α=0.05作为检验水准。
2 结果 2.1 各组大鼠肝功能指标第1~4周,模型组大鼠血清中ALT、AST和TB水平高于其他2组(P < 0.05),而阻断组与对照组上述指标比较差异无统计学意义(P>0.05)。第2~4周,模型组大鼠血清中ALB和AcSDKP水平低于其他2组(P < 0.05),而阻断组和对照组上述指标比较差异无统计学意义(P>0.05)。见表 1。
| (n=15, x±s) | |||||||
| Group | ALT[λB/(U·L-1)] | AST[λB/(U·L-1)] | |||||
| (week) 1 | 2 | 4 | 1 | 2 | 4 | ||
| Control | 32.5±6.7 | 29.8±5.3 | 26.7±7.1 | 51.6±8.6 | 45.7±8.6 | 32.5±8.4 | |
| Model | 92.6±10.4*△ | 242.5±15.6*△ | 488.9±22.3*△ | 109.4±14.6*△ | 198.3±9.7*△ | 365.1±24.8*△ | |
| Blocking | 37.4±6.2 | 33.4±4.8 | 25.9±6.3 | 54.7±7.3 | 42.1±7.7 | 30.8±7.5 | |
| Group | TB[cB/(μmol·L-1)] | ALB[ρB/(g·L-1)] | |||||
| (week) 1 | 2 | 4 | 1 | 2 | 4 | ||
| Control | 0.4±0.1 | 0.4±0.1 | 0.9±0.2 | 39.0±5.5 | 38.1±4.9 | 37.9±5.6 | |
| Model | 7.2±1.6*△ | 9.8±2.1*△ | 15.7±3.2*△ | 38.3±4.1 | 23.4±3.5*△ | 18.2±2.9*△ | |
| Blocking | 0.5±0.2 | 0.5±0.1 | 0.8±0.1 | 38.6±5.1 | 36.7±4.6 | 36.6±5.0 | |
| Group | AcSDKP(nmol·mg-1) | ||||||
| (week) 1 | 2 | 4 | |||||
| Control | 0.90±0.13 | 0.85±0.14 | 0.79±0.12 | ||||
| Model | 0.86±0.12 | 0.66±0.10*△ | 0.41±0.09*△ | ||||
| Blocking | 0.88±0.13 | 0.79±0.12 | 0.74±0.10 | ||||
| * P < 0.05 compared with control group; △ P < 0.05 compared with blocking group. | |||||||
第1~4周,3组大鼠肝组织中PCⅢ、CⅣ和HA水平比较差异无统计学意义(P>0.05);第2~4周,模型组PCⅢ、CⅣ和HA水平高于其他2组(P < 0.05),而阻断组和对照组上述各指标比较差异无统计学意义(P>0.05)。见表 2。
| [n=15, x±s, ρB/(μg·L-1)] | |||||||
| Group | PCⅢ | CⅣ | |||||
| (week) 1 | 2 | 4 | 1 | 2 | 4 | ||
| Control | 1 035.8±186.1 | 1 087.4±165.6 | 1 062.3±137.8 | 997.5±89.7 | 982.6±125.3 | 1 031.2±168.5 | |
| Model | 1 102.2±209.5 | 1 679.4±311.0*△ | 1 863.2±264.7*△ | 989.6±102.3 | 1 728.8±197.4*△ | 2 136.8±237.3*△ | |
| Blocking | 1041.6±174.3 | 1099.1±172.1 | 1077.6±142.2 | 1001.2±102.6 | 988.9±102.1 | 1042.0±154.9 | |
| Group | HA | ||||||
| (week)1 | 2 | 4 | |||||
| Control | 879.6±90.3 | 887.2±78.3 | 869.8±65.7 | ||||
| Model | 869.4±87.5 | 1099.6±97.4*△ | 1356.2±93.1*△ | ||||
| Blocking | 883.4±92.9 | 896.4±71.6 | 876.9±62.0 | ||||
| * P < 0.05 compared with control group; △ compared with blocking group. | |||||||
第1周,各组大鼠肝细胞和肝组织结构完整、基本正常,无明显坏死细胞及炎性细胞浸润、纤维组织增生现象。第2周,模型组大鼠肝组织呈现小灶状坏死,小叶结构基本完整,汇管区呈现少量纤维增生以及炎性细胞浸润,肝细胞大量发生脂肪变性;对照组及阻断组大鼠肝细胞和肝组织基本正常。第4周,模型组大鼠肝组织呈片状坏死,肝小叶破坏,结构紊乱,假小叶,汇管区扩大,较多炎性细胞浸润,大量纤维组织增生,肝细胞脂肪变性程度更加严重;对照组和阻断组肝细胞及肝组织基本正常。见图 1(插页四)。
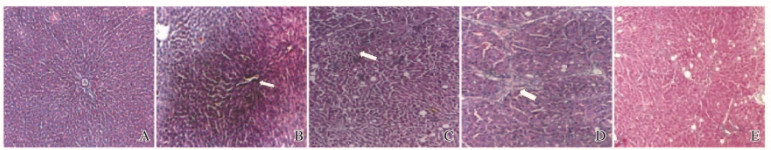
|
| A:Control group (1 week); B-D: Model group (1, 2, and 4 weeks); E: Blocking group (1 week). 图 1 各组大鼠肝组织HE染色结果(×100) Figure 1 HE staining results of liver tissue of rats in various groups (×100) |
|
|
第2周开始,模型组大鼠肝组织中胸腺素β4及ACE水平高于其他2组,而POP水平低于其他2组(P < 0.05);阻断组与对照组上述指标比较差异无统计学意义(P>0.05)。见表 3。
| (n=5, x±s) | |||||||||||
| Group | Tβ4 | POP | ACE | ||||||||
| (week) 1 | 2 | 4 | 1 | 2 | 4 | 1 | 2 | 4 | |||
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Model | 1.018 | 1.746*△ | 3.157*△ | 1.025 | 0.828*△ | 0.535*△ | 1.009 | 1.864*△ | 2.036*△ | ||
| Blocking | 1.009 | 1.012 | 1.012 | 1.019 | 0.998 | 0.987 | 1.005 | 1.009 | 1.003 | ||
| * P < 0.05 compared with control group; △ P < 0.05 compared with blocking group. | |||||||||||
近年来AcSDKP在临床上被广泛关注,既往研究[6]显示:AcSDKP具有较强抑制巨噬细胞、肥大细胞等炎性细胞浸润,拮抗成纤维细胞增殖及胶原合成的作用,因而在体内表现出明显的抗炎及抗纤维化效应。研究[7-8, 14-15]证实:内源性AcSDKP生成主要依靠POP分解胸腺素β4产生,而其降解主要依靠ACE分解,这些AcSDKP生成与降解系统均完整地存在于肝内。生理学研究[9, 16]也证实:AcSDKP在肝内浓度接近于心脏和肾脏。动物实验[7-12]显示:大鼠心脏组织ACE活性上调或抑制POP活性,均降低心脏和/或肾脏组织中内源性AcSDKP水平,导致心脏纤维化和/或肾小球硬化。实验动物持续注射外源性AcSDKP能有效抑制高血压、心脏缺血再灌注、高血压肾损害和糖尿病肾损害中出现的靶器官纤维化[10-11, 13-14]。最近研究[12, 15]显示:AcSDKP能有效介导ACE抑制剂的抗心、肾纤维化效应。与在体研究结果类似,离体研究[13, 16]也显示:AcSDKP能有效抗心脏成纤细胞和肾小球系膜细胞的纤维化活性。
虽然AcSDKP在肝内的生理作用目前未明确,但是根据以上相关研究背景,可以推测内源性AcSDKP可能参与对肝脏纤维化及细胞外基质自稳定的调控[17-18]。最近研究[1, 4]显示:机体多种器官及组织中广泛存在胸腺素β4-POP-AcSDKP轴,且发挥重要生物学功能。有研究者在胆总管结扎和四氯化碳注射等大鼠肝纤维化模型中,ACEI(如卡托普利等)已被证实对肝纤维化有保护作用,推测其作用机制可能与上调AcSDKP有关[19-20]。但经典大鼠肝纤维化模型中内源性AcSDKP水平动态变化及调控因子(如胸腺素β4、POP和ACE等)改变情况,目前尚无临床研究进行阐述,因此本研究通过选择胆总管结扎大鼠肝纤维化模型来探讨此病理生理过程[21-23]。
本研究结果显示:通过胆总管结扎制备大鼠肝纤维化模型病理生理模拟程度较好,大鼠的肝功能明显降低,肝纤维化指标明显升高,病理检查也支持肝纤维化病理发生,该病理过程在结扎胆总管后第2周显现,这一结果与既往研究[19-20]结论相似。而模型组大鼠肝组织中AcSDKP水平也是在第2周明显降低,与肝纤维化保持同步,证实内源性AcSDKP水平减少会诱导肝脏纤维化发生。同时模型组大鼠肝组织中POP水平明显降低、胸腺素β4水平明显增加,提示内源性AcSDKP生成降低;而肝组织中ACE水平明显升高,提示内源性AcSDKP降解增加,导致机体内肝组织内源性AcSDKP水平在第2周明显降低。本研究通过应用AcSDKP特异性水解酶阻断剂ACEI来提高动物模型体内的内源性AcSDKP水平,结果显示:大鼠肝脏纤维化程度明显改善,这也证实内源性AcSDKP水平降低可能是胆总管结扎致使大鼠发生肝纤维化的重要原因之一。
综上所述,胆总管结扎大鼠肝纤维化所致内源性AcSDKP水平明显降低有可能是通过Tβ4-POP-AcSDKP轴实现的,对于Tβ4-POP-AcsDKP轴致使内源性AcsDKP缺乏的具体机制在今后的研究中值得进一步探讨。
| [1] | Kanasaki K, Nagai T, Nitta K, et al. N-acetyl-seryl-aspartyl-lysyl-proline:a valuable endogenous anti-fibrotic peptide for combating kidney fibrosis in diabetes[J]. Front Pharmacol, 2014, 5: 70–81. |
| [2] | Hrenak J, Paulis L, Simko F. N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP):Potential target molecule in research of heart, kidney and brain[J]. Curr Pharm Des, 2015, 21(35): 5135–5143. DOI:10.2174/1381612821666150909093927 |
| [3] | Mnguni AT, Engel ME, Borkum MS, et al. The effects of angiotensin converting enzyme inhibitors (ACE-I) on human N-Acetyl-Seryl-Aspartyl-Lysyl-Proline (Ac-SDKP) levels:a systematic review and Meta-Analysis[J]. PLoS One, 2015, 10(12): e0143338. DOI:10.1371/journal.pone.0143338 |
| [4] | Mnguni AT, Engel ME, Borkum MS, et al. The Effects of Angiotensin Converting Enzyme Inhibitors (ACE-I) on Human N-Acetyl-Seryl-Aspartyl-Lysyl-Proline (Ac-SDKP) Levels:A Systematic Review and Meta-Analysis[J]. PLoS One, 2015, 10(12): e0143338. DOI:10.1371/journal.pone.0143338 |
| [5] | 刘晓亚, 刘瑞霞, 崔立建, 等. 胆管结扎和四氯化碳诱导Wistar大鼠肝纤维化模型的建立及相关指标的对比分析[J]. 临床肝胆病杂志, 2015, 31(2): 219–224. DOI:10.3969/j.issn.1001-5256.2015.02.018 |
| [6] | Chen YW, Liu BW, Zhang YJ, et al. Preservation of basal AcSDKP attenuates Carbon tetrachloride-induced fibrosis in the rat liver[J]. J Hepatol, 2010, 53(3): 528–536. DOI:10.1016/j.jhep.2010.03.027 |
| [7] | Yuan J, Shen Y, Yang X, et al. Thymosin β4 alleviates renal fibrosis and tubular cell apoptosis through TGF-β pathway inhibition in UUO rat models[J]. BMC Nephrol, 2017, 18(1): 314–324. DOI:10.1186/s12882-017-0708-1 |
| [8] | Zhu L, Cheng M, Liu Y, et al. Thymosin-β4 inhibits proliferation and induces apoptosis of hepatic stellate cells through PI3K/AKT pathway[J]. Oncotarget, 2017, 8(40): 68847–68853. |
| [9] | Deng H, Xu H, Zhang X, et al. Protective effect of Ac-SDKP on alveolar epithelial cells through inhibition of EMT via TGF-β1/ROCK1 pathway in silicosis in rat[J]. Toxicol Appl Pharmacol, 2016, 294: 1–10. DOI:10.1016/j.taap.2016.01.010 |
| [10] | Mitrovic V, Seferovic P, Dodic S, et al. Cardio-renal effects of the A1 adenosine receptor antagonist SLV320 in patients with heart failure[J]. Circ Heart Fail, 2009, 2(6): 523–531. DOI:10.1161/CIRCHEARTFAILURE.108.798389 |
| [11] | Stienstra R, Saudale F, Duval C, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity[J]. Hepatology, 2010, 51(2): 511–522. DOI:10.1002/hep.23337 |
| [12] | Hu Q, Li J, Nitta K, et al. FGFR1 is essential for N-acetyl-seryl-aspartyl-lysyl-proline regulation of mitochondrial dynamics by upregulating microRNA let-7b-5p[J]. Biochem Biophys Res Commun, 2018, 495(3): 2214–2220. DOI:10.1016/j.bbrc.2017.12.089 |
| [13] | Masuyer G, Douglas RG, Sturrock ED, et al. Structural basis of Ac-SDKP hydrolysis by Angiotensin-Ⅰ converting enzyme[J]. Sci Rep, 2015, 5: 13742. DOI:10.1038/srep13742 |
| [14] | Srivastava SP, Shi S, Kanasaki M, et al. Effect of antifibrotic MicroRNAs crosstalk on the action of n-acetyl-seryl-aspartyl-lysyl-proline in diabetes-related kidney fibrosis[J]. Sci Rep, 2016, 6: 29884. DOI:10.1038/srep29884 |
| [15] | Li J, Shi S, Srivastava SP, et al. FGFR1 is critical for the anti-endothelial mesenchymal transition effect of N-acetyl-seryl-aspartyl-lysyl-proline via induction of the MAP4K4 pathway[J]. Cell Death Dis, 2017, 8(8): e2965. DOI:10.1038/cddis.2017.353 |
| [16] | 闫静波. AcSDKP对硅肺纤维化大鼠胶原含量及NF-κBp65表达的影响[J]. 重庆医学, 2013(26): 3139–3141. DOI:10.3969/j.issn.1671-8348.2013.26.024 |
| [17] | Conte E, Fagone E, Gili E, et al. Preventive and therapeutic effects of thymosin β4 N-terminal fragment Ac-SDKP in the bleomycin model of pulmonary fibrosis[J]. Oncotarget, 2016, 7(23): 33841–33854. |
| [18] | Liao TD, Nakagawa P, Janic B, et al. N-Acetyl-Seryl-Aspartyl-Lysyl-Proline:mechanisms of renal protection in mouse model of systemic lupus erythematosus[J]. Am J Physiol Renal Physiol, 2015, 308(10): F1146–F1154. DOI:10.1152/ajprenal.00039.2015 |
| [19] | Ma X, Yuan Y, Zhang Z, et al. An analog of Ac-SDKP improves heart functions after myocardial infarction by suppressing alternative activation (M2) of macrophages[J]. Int J Cardiol, 2014, 175(2): 376–378. DOI:10.1016/j.ijcard.2014.05.016 |
| [20] | Monge M, Paquet V, Bergerot D, et al. Dose-effect relationship of perindopril 10, 14 and 20mg assessed by urine and plasma AcSDKP levels in mildly sodium-depleted healthy volunteers[J]. Int J Cardiol, 2016, 222: 648–653. DOI:10.1016/j.ijcard.2016.07.164 |
| [21] | 牛学敏, 王宝玉, 王洋, 等. PTEN在CCl_4诱导肝纤维化大鼠模型中的作用及益气活血方对其的影响[J]. 临床肝胆病杂志, 2018, 34(1): 122–128. |
| [22] | Jiang Y, Han T, Zhang ZG, et al. Potential role of thymosin beta 4 in the treatment of nonalcoholic fatty liver disease[J]. Chronic Dis Transl Med, 2017, 3(3): 165–168. DOI:10.1016/j.cdtm.2017.06.003 |
| [23] | Hong Y, Yao Q, Zheng L. Thymosin β4 attenuates liver fibrosis via suppressing Notch signaling[J]. Biochem Biophys Res Commun, 2017, 493(4): 1396–1401. DOI:10.1016/j.bbrc.2017.09.156 |
 2018, Vol. 44
2018, Vol. 44


