肝细胞肝癌(hepatocellular carcinoma,HCC)是常见的恶性肿瘤之一,病死率较高。18F-FDG PET/CT显像常用于恶性肿瘤的诊断、临床分期及预后评估,但其诊断原发HCC的灵敏度不高,假阴性率可达40%~50%[1]。18F-FDG与11C-乙酸盐PET/CT显像具有一定程度的互补性,常用于HCC的诊断[2]、临床分期[3-4]及肝脏肿瘤的鉴别诊断[5-6]。先前关于18F-FDG及11C-乙酸盐双示踪剂在HCC患者中的应用研究中包含了低、中、高分化的HCC 3种不同分化类型,并未对特定的病理类型选择不同示踪剂进行显像[2, 7-8]。笔者仅对中、高分化HCC患者进行双示踪剂显像,初步探讨11C-乙酸盐单示踪剂PET/CT显像能否取代双示踪剂显像用于监测中、高分化HCC患者的复发与转移。
1 资料与方法 1.1 一般资料回顾性分析从2015年1月至2016年12月于我院行11C-乙酸盐和18F-FDG PET/CT躯干显像的10例中、高分化HCC患者。入选患者均为男性,年龄27~74岁,平均年龄(55.5±13.7)岁。所有患者均为手术或介入治疗后6~20个月的HCC患者,其中6例为中分化HCC,4例为高分化HCC。纳入标准:中、高分化HCC患者在病情监测过程中发现甲胎蛋白水平升高,临床怀疑复发或转移。排除标准:低分化HCC患者。入选患者一周内行18F-FDG和11C-乙酸盐PET/CT检查。所有患者最终通过病理或我院影像学检查确诊转移或复发。所有患者或家属对本研究均知情并签署了知情同意书。
1.2 检查方法18F-FDG和11C-乙酸盐均为我科合成,放化纯度大于95%,按中国药典标准规定行24 h细菌培养及凝胶法细菌内毒素检测(次日检测),结果均为阴性。患者空腹4~6 h,在安静环境中休息10~15 min,经静脉注射2.96~4.44 MBq/kg 18F-FDG后安静休息45~60 min,使用德国西门子Biograph 64型PET/CT行5床位躯干采集。CT的管电流为100 mAs,管电压为120 kV,旋转时间为0.5 s,螺距为0.9,层厚为5 mm。CT图像用于随后PET衰减校正。PET采用三维发射采集,2 min/床位。PET重建采用点扩散技术的迭代法(TureX),使用3次迭代和21个子集,图像矩阵为172 mm×172 mm,4 mm半高宽的高斯滤波,加散射校正。18F-FDG检查后1周内行11C-乙酸盐PET/CT检查。患者空腹4~6 h,经静脉注射2.96~4.44 MBq/kg 11C-乙酸盐,5 min后使用上述相同设备和方法行躯干采集。
1.3 图像分析由2位高级职称核医学医师在不知患者病理分级的情况下对PET/CT图像进行分析并测定病灶及本底SUVmax。SUVmax取2位医师所得数值的平均值。PET图像上代谢高于正常肝脏组织的病灶判定为阳性,低于或类似于正常肝脏组织的病灶判定为阴性。如2位医师意见不一致则通过共同讨论判定病灶阳性或阴性。PET显像未见放射性异常浓聚者,根据同机CT或其他影像学资料确定病灶位置,用视觉融合的方法在相对应部位勾画与病灶相同大小的ROI。
2 结果10例中、高分化HCC患者的临床资料及11C-乙酸盐、18F-FDG显像结果见表 1。11C-乙酸盐显像发现18个阳性病灶,灵敏度为100%(18/18);18F-FDG显像发现5个阳性病灶,灵敏度为27.8%(5/18);两种示踪剂同时发现5个阳性病灶(在4例中分化HCC患者中),11C-乙酸盐显像探测病灶的灵敏度与双示踪剂联合显像相同。18F-FDG显像发现2例中分化(图 1)和4例高分化HCC(图 2)病灶显像为阴性。11C-乙酸盐显像病灶的SUVmax为1.3~14.2,靶/本底比值(the ratio of target to background,T/B)为1.1~14.3;18F-FDG显像病灶的SUVmax为0.5~3.4,T/B为0.6~1.1。8例患者(13个病灶)病理证实为肿瘤复发或转移,2例患者(5个病灶)3个月后我院复查PET/CT或CT证实为转移。
| 表1 10例男性中、高分化肝细胞肝癌患者的临床资料及11C-乙酸盐、18F-FDG PET/CT显像结果 Table 1 Clinical data and 11C-acetate and 18F-FDG PET/CT results of 10 male cases of moderately and highly differentiated hepatocellular carcinoma |
|
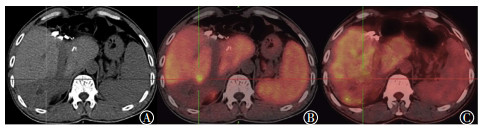
图 1 中分化肝细胞肝癌患者的11C-乙酸盐及18F-FDG PET/CT显像图 Figure 1 11C-acetate and 18F-FDG PET/CT imaging in patient with moderately differentiated hepatocellular carcinoma 患者男性, 51岁, 肝右后叶切除术后半年, AFP进行性增高。图中, A:CT横断位显示术区片状低密度影(十字线所示); B:11C-乙酸盐PET/CT融合图横断位显示切缘局限性浓聚灶(十字线所示); C:18F-FDG PET/CT融合图横断位显示切缘相同位置无明显异常放射性摄取(十字线所示)。FDG:氟脱氧葡萄糖; PET/CT:正电子发射断层显像计算机体层摄影术; AFP:甲胎蛋白。 |
|
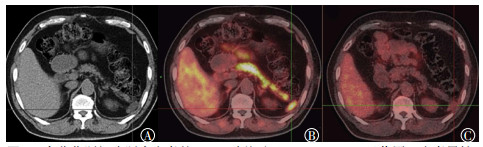
图 2 高分化肝细胞肝癌患者的11C-乙酸盐及18F-FDG PET/CT显像图 Figure 2 11C-acetate and 18F-FDG PET/CT imaging in patient with highly differentiated hepatocellular carcinoma 患者男性, 63岁, 肝左叶肿瘤切除术后1年, AFP进行性增高。图中, A:CT横断位显示胰尾外侧实性结节(十字线所示); B:11C-乙酸盐PET/CT融合图横断位显示结节异常放射性浓聚(十字线所示); C:18F-FDG PET/CT融合图横断位显示结节无明显异常放射性摄取(十字线所示)。FDG:氟脱氧葡萄糖; PET/CT:正电子发射断层显像计算机体层摄影术; AFP:甲胎蛋白。 |
HCC在我国是常见的恶性肿瘤之一。及时且准确地判断HCC治疗后的残存、复发或转移情况直接关系到临床治疗方案的选择及患者预后。18F-FDG PET/CT显像在大多数恶性肿瘤的疗效监测及评估中发挥着越来越大的作用,但对HCC原发灶的诊断效果不佳,尤其是在高分化HCC患者中。18F-FDG PET/CT显像中18F-FDG在细胞内的浓聚程度取决于细胞内磷酸激酶活性和葡萄糖-6-磷酸酶活性之比,分化较好的肿瘤细胞内含有较高浓度的葡萄糖-6-磷酸酶,去磷酸化水平高,可以加速18F-FDG的细胞转出过程[9],因此,高分化HCC病灶细胞内18F-FDG的含量较低,常表现为假阴性。在对HCC转移灶的18F-FDG PET/CT显像研究中,Park等[4]发现18F-FDG比11C-乙酸盐更具优势;而Ho等[7]的研究结果表明对HCC转移灶应用11C-乙酸盐单示踪剂显像似乎有一定的优势。本研究结果与Ho等[7]的研究结果大致相同,在高分化HCC中,11C-乙酸盐显像发现8个病灶,而18F-FDG显像均为假阴性;在中分化HCC中,18F-FDG显像的阳性率也仅为50%,因此,对中、高分化HCC转移及复发病灶应用11C-乙酸盐与18F-FDG双示踪剂显像并没有明显的优势。
11C-乙酸盐作为氨基酸及甾醇合成的前体,可以从另一方面反映肿瘤代谢情况不受葡萄糖去磷酸化的影响,是一种很有潜力的正电子显像剂,已广泛应用于多种高分化、低度恶性的肿瘤显像中,可弥补18F-FDG显像的不足。乙酸盐参与细胞脂代谢,恶性肿瘤细胞脂类代谢比正常细胞活跃,11C-乙酸盐进入体内后较多地进入癌细胞中,其进入量与反映肿瘤增殖程度的脂肪及磷脂膜合成量呈正相关[10-11]。此外,11C-乙酸盐显像还可用于前列腺癌[12]及肾脏肿瘤[13]的诊断及分期。本研究中11C-乙酸盐PET/CT显像10例患者均为阳性,且总共发现18个病灶,对中、高分化HCC的复发与转移的探测灵敏度明显高于18F-FDG PET/CT显像,与双示踪剂联合显像灵敏度相同。
本研究仅针对治疗后高度怀疑复发与转移的中、高分化HCC患者进行双示踪剂显像,并未纳入低分化HCC患者。研究结果表明11C-乙酸盐显像可显著提高中、高分化HCC的复发与转移诊断的灵敏度,且推荐使用11C-乙酸盐显像用于监测中、高分化HCC的复发与转移。本研究的不足之处在于病例数相对较少,笔者所在课题组的下一步工作将继续对现有结论作进一步验证。
利益冲突 本研究由署名作者按以下贡献声明独立开展,不涉及任何利益冲突。
作者贡献声明 麻广宇负责研究命题提出、设计及试验,以及论文起草;刘家金负责数据采集、处理及技术支持;徐白萱及陈英茂负责试验指导;张晓军及刘健负责药物制备;张锦明负责命题提出及设计,试验指导及论文起草、最终版修订。
| [1] | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses[J]. J Nucl Med, 2003, 44(2): 213–221. |
| [2] | Larsson P, Arvidsson D, Björnstedt M, et al. Adding 11C-acetate to 18F-FDG at PET Examination Has an Incremental Value in the Diagnosis of Hepatocellular Carcinoma[J]. Mol Imaging Radionucl Ther, 2012, 21(1): 6–12. DOI:10.4274/Mirt.87 |
| [3] | Cheung TT, Ho CL, Lo CM, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria:surgeon's perspective[J]. J Nucl Med, 2013, 54(2): 192–200. DOI:10.2967/jnumed.112.107516 |
| [4] | Park JW, Kim JH, Kim SK, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma[J]. J Nucl Med, 2008, 49(12): 1912–1921. DOI:10.2967/jnumed.108.055087 |
| [5] | Huo L, Dang Y, Lv J, et al. Application of dual phase imaging of 11C-acetate positron emission tomography on differential diagnosis of small hepatic lesions[J/OL]. PLoS One, 2014, 9(5): e96517[2018-01-12]. https: //www. ncbi. nlm. nih. gov/pmc/articles/PMC4015995. DOI: 10.1371/journal.pone.0096517. |
| [6] | Magini G, Farsad M, Frigerio M, et al. C-11 acetate does not enhance usefulness of F-18 FDG PET/CT in differentiating between focal nodular hyperplasia and hepatic adenoma[J]. Clin Nucl Med, 2009, 34(10): 659–665. DOI:10.1097/RLU.0b013e3181b53488 |
| [7] | Ho CL, Chen S, Cheng TK, et al. PET/CT characteristics of isolated bone metastases in hepatocellular carcinoma[J]. Radiology, 2011, 258(2): 515–523. DOI:10.1148/radiol.10100672 |
| [8] | Ho CL, Chen S, Yeung DW, et al. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma[J]. J Nucl Med, 2007, 48(6): 902–909. DOI:10.2967/jnumed.106.036673 |
| [9] | Yoon KT, Kim JK, Kim DY, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in detecting extrahepatic metastasis in pretreatment staging of hepatocellular carcinoma[J]. Oncology, 2007, 72. DOI:10.1159/000111715 |
| [10] | Yoshimoto M, Waki A, Yonekura Y, et al. Characterization of acetate metabolism in tumor cells in relation to cell proliferation:acetate metabolism in tumor cells[J]. Nucl Med Biol, 2001, 28(2): 117–122. DOI:10.1016/S0969-8051(00)00195-5 |
| [11] | Salem N, Kuang Y, Corn D, et al. [(Methyl)1-11C]-acetate metabolism in hepatocellular carcinoma[J]. Mol Imaging Biol, 2011, 13(1): 140–151. DOI:10.1007/s11307-010-0308-y |
| [12] | Polanec SH, Andrzejewski P, Baltzer PAT, et al. Multiparametric[11C]Acetate positron emission tomography-magnetic resonance imaging in the assessment and staging of prostate cancer[J/OL]. PLoS One, 2017, 12(7): e0180790[2018-01-12]. https: //www. ncbi. nlm. nih. gov/pmc/articles/PMC5515396. DOI: 10.1371/journal.pone.0180790. |
| [13] | Ho CL, Chen S, Ho KM, et al. Dual-tracer PET/CT in renal angiomyolipoma and subtypes of renal cell carcinoma[J]. Clin Nucl Med, 2012, 37(11): 1075–1082. DOI:10.1097/RLU.0b013e318266-cde2 |
 2018, Vol. 42
2018, Vol. 42


