2 南方海洋科学与工程广东省实验室(珠海), 广东 珠海 519000;
3 广东省地质过程与矿产资源探查重点实验室, 广东 广州 510275)
地球系统的物质循环制约着地球表层资源环境问题的形成和发展[1]。陆地水域(河流、湖泊、沼泽等)作为地表过程产物的接纳容器,在地球表层系统物质循环和能量流动过程中起着重要作用[2~4]。Aufdenkampe等[5]估算全球陆地水体排向大气的二氧化碳(CO2)通量为3.28 PgC/a,其中湖泊水库0.64 PgC/a,河溪0.56 PgC/a,沼泽2.08 PgC/a。筑坝形成“蓄水河流”(下称水库)改变了自然河流的碳循环过程[6~7],一方面蓄水淹没土地减少了陆地生态系统对大气CO2的吸收,同时淹没的有机物在水中被矿化,可能使水库成为比火电站更甚的温室气体排放源[8~10];另一方面沉积在库底的陆地有机物分解缓慢,部分有机碳将长期埋藏于库底构成一个相对稳定的碳汇[4]。此外,水库水-气界面温室气体排放随库龄增长呈递减趋势[11~13]。Prairie等[14]研究表明,水库累积CO2排放通量的25 %来自水库运行过程;Barros等[12]依据全球85个水库的数据估计水库以CO2形式向大气释放0.048 PgC/a,以甲烷(CH4)形式向大气释放0.003 PgC/a,相当于全球内陆水体碳排放的4 %;Deemer等[15]估算全球水库表面CO2通量为0.5~1.2 PgC/a,且其分布存在气候带差异,热带地区湖泊水库CO2通量(0.45 PgC/a)远大于温带(0.08 PgC/a)和寒带(0.11 PgC/a)[5]。然而,沉积埋藏将颗粒碳沉于库底可抵消一部分碳排放。假设大坝截留的悬浮物中颗粒有机碳(POC)的最低含量为0.5 %,则水库沉积埋藏可使CO2总排放量降低约33 % [16]。据估算,乌江渡水库沉积物的净碳汇为23. 9 g/(m2·a)[17]。研究发现,部分水库由于生物量和有机质含量较高,水-气界面CO2交换通量较大[16, 18~22],部分水库水体有机质含量较低,且库区水体光合作用强于矿化作用,成为大气CO2的汇[23~25]。截至目前,水库碳循环机制尚未深入研究,由于实测数据较少,关于水库CO2的“源/汇”问题仍存在不确定性和争议[7~8, 21, 26~27]。水库水-气界面CO2交换量受水体二氧化碳分压(pCO2)制约。本文将综述水库水体pCO2数据获取方法、时空分布特点及其影响因素,提出未来陆地水域pCO2研究的可能关注问题。
1 水库碳的迁移、转化20世纪50年代以来,全球河流系统受到人类污染和拦截调蓄的共同影响。根据国际大坝委员会2018年统计,全球大坝共计59071座[28]。河流建坝将异养的自然河流转变成自养的水库,从而改变河流的水文情势和它的物理、化学和生物学特征及动态,进而影响到生源要素碳的生物地球化学行为[29]。
水库碳的来源分为内源和外源两类,主要存在形式有:溶解有机碳(DOC)、溶解无机碳(DIC)、POC和颗粒无机碳(PIC)4种。其中DIC主要源自:1)流域碳酸盐矿物的风化;2)土壤CO2;3)水库浮游植物呼吸作用和有机质矿化;4)大气降水(对河流DIC贡献很小,可忽略)[30]。
水库中不同形式的碳迁移、转化过程包括POC和PIC的沉降、POC和DOC的微生物矿化、浮游植物生长吸收DIC以及随水库水体的排泄输出(图 1)。水库蓄水后出现浮游植物生长使水库有机碳含量增加;埋藏作用延长碳循环过程[6];独特的水文特征促进其内部微生物呼吸作用,易产生温室气体[31];出库泄水时卸荷作用导致水库下游河道CO2排放增加[32]等现象。

|
图 1 水库碳生物地球化学循环示意图(改自文献[4, 6~7]) Fig. 1 Carbon biogeochemical cycle in reservoir, modified from references[4, 6~7] |
水库CO2的释放途径有3种[33]:1)水库表面扩散,指CO2气体经由水-气界面扩散至大气,是水库CO2的最主要释放途径[31, 33~34];2)冒泡排放,指沉积物-水界面产生的气泡通过水柱进入大气,主要发生在水库蓄水的最初2年和水库表面以下10 m内[35];3)涡轮机、溢洪道和大坝下游河流脱气排放。水库大坝前后水位高程悬殊,水体通过涡轮机溢洪道时因压力骤减而排放大量气体。研究表明从涡轮机和溢洪道释放的CO2约占水库总排放量的0~16 % [18, 36]。大坝下游河流水体CH4和CO2气体含量较高,排放的CO2约占总排放量的1.63~40 % [18, 36~37]。
上述排放方式中的冒泡排放,由于CO2易于溶解(一个标准大气压和25 ℃条件下,1 L水可以溶解约0.8 L CO2),水库底部CO2向上运移过程中很容易被水吸收,其气泡释放通量常小于总通量的1 % [18, 33],故计算水库水-气界面CO2交换通量时可被忽略。水-气界面CO2交换通量可用分子扩散模型计算,受水体与大气间pCO2之差,气体交换系数等因素影响,气体交换系数又受流速、风速、温度影响。计算公式如下[38~39]:
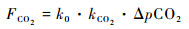
|
(1) |
公式(1)中,FCO2为水-气界面CO2交换通量(mmol/(m2·d));k0为某气温下CO2溶解度(mol/L·atm),大小受到水体温度、盐度、压强等因素的影响;kCO2为CO2交换速率(cm/h),目前关于kCO2的选取与估算仍存在不确定性,在海-气FCO2研究中广泛应用Wanninkhof模式[40],在湖泊、水库研究中Cole模式[41]以及Upstill模式[42]应用较多,上述计算模式均可通过U(采样时间点的平均风速(m/s))和Sc(t ℃下CO2的Schmidt常数)两参数来计算kCO2;ΔpCO2为水体与大气pCO2之差。
2 水体pCO2数据获取水体pCO2数据的获取方法有现场直接测量和利用CO2在水溶液中的碳酸平衡原理和亨利定律间接计算两种,其中计算方法在水库中应用较为广泛(表 1),这是因为陆地水体物理、化学和生物过程的多变性以及人为干扰使得pCO2时空差异较大,不易直接测量[43]。
| 表 1 水库主要特征 Table 1 The main features of the studied reservoirs |
水体pCO2的测量方法如下:
(1) 顶空平衡法(摇瓶法)。摇动封闭瓶子或注射器中收集的水样,促进水样和顶空气之间pCO2的平衡。待其稳定后,利用气体注射器对平衡空气进行采样,然后用气相色谱仪(GC)或红外气体分析器(IRGA)对CO2浓度进行分析。摇瓶法一直作为pCO2直接测量的标准方法被大范围应用,但手动平衡的误差及非连续采样的特点限制其在pCO2时空变化差异较大的内陆水域应用[61~62]。
(2) 平衡器法。应用最广泛的两种方法是喷淋式平衡器法和弹珠式平衡器法。通过在垂直玻璃树脂管中喷射水滴或装满玻璃球增加水气接触面积,在容器顶部设置平衡室,以便快速达到气液平衡并抽气监测。在充足稳定的电力保证泵和分析器运行条件下,平衡器法可获得连续高频的pCO2,但电力供应和维护使其难以在某些内陆河流中持续监测pCO2[54, 61]。
(3) 静态箱法。自制一塑料箱,箱内壁安装温度计测定箱体温度,外部套上厚泡沫板并包上锡纸,防止阳光照射影响。测量时,先将静态箱提起,使箱内气压与大气压保持平衡,然后将箱子缓慢放于水体表面,使其保持静止状态,接通分析仪,记录pCO2数值等[44]。静态箱法无需依靠电力获取平衡且所需成本低,可在各种陆地水域多点重复部署,但是该方法存在响应时间长、生物絮凝等问题[61]。
2.2 水体pCO2的间接计算理论上,DIC由溶解态CO2(aq)、碳酸(H2CO3)、重碳酸盐(HCO3-)和碳酸根离子(CO32-)组成,水溶液平衡时各组分在水体中的浓度主要与pH、水温和离子强度有关[41]。若已测出水体pH值、水温和阴阳离子强度,则根据CO2在水溶液中的碳酸平衡原理和亨利定律计算pCO2(μatm)[19, 48]:
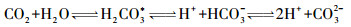
|
(2) |
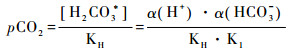
|
(3) |
淡水中HCO3-构成碱度(Alk)的99 % [63]。根据原位测量的水温,选择DIC、pH和Alk中任意两个参数用热力学平衡方程可计算pCO2(μatm)[55]:
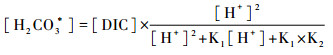
|
(4) |
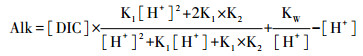
|
(5) |
公式(2~5)中Ki为平衡常数,KH代表亨利常数;α(H+)和α(HCO3-)分别表示H+和HCO3-的活度;KW代表水体有关温度的解离常数。
目前水体pCO2数据的获取多采用计算方法(表 1),但是各种计算结果存在较大差异。例如,采用Alk和pH值估算的pCO2数值比采用DIC和pH估算的值高约12 % ~66 %;非碳酸盐体系碱度的存在使得pCO2计算值高约40 % [63]。此外,获取各参数的化学分析方法存在仪器及操作误差,导致计算结果存在差异[61, 64]。
3 水库pCO2时空特征水库水体除发生自然生物地球化学过程外,还受到人为过程的干扰,导致pCO2存在较大时空差异[55]。
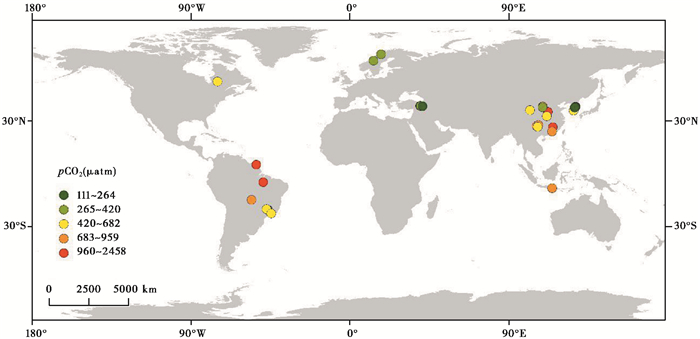
3.1 空间变化特征水库二氧化碳分压的空间变化特征主要表现为水库间的差异与水库内部的差异。迄今关于水库表层水体pCO2的研究多集中于亚洲东部、南美东部、北美及欧洲西部(图 2)等地,研究表明,水库pCO2变化于111~2457 μatm之间,平均为744 μatm;其中70.97 %的水库(n=31)表层水体pCO2高于大气pCO2 (400 μatm[21]),大约是河流pCO2 (3100 μatm)[3]的四分之一。不同气候带水库表层水体pCO2也略有差异,表现出热带(969 μatm)>温带(695 μatm)>寒温带(468 μatm)的趋势,与Raymond等[3]和Wen等[21]的研究结果一致。
|
图 2 水库表层水体pCO2分布图(数据源自文献[8, 20, 22, 24, 35, 38, 44~45, 47, 49~50, 53, 55~60]) Fig. 2 Global spatial distribution of pCO2 on the reservoir surface, data derived from references [8, 20, 22, 24, 35, 38, 44~45, 47, 49~50, 53, 55~60] |
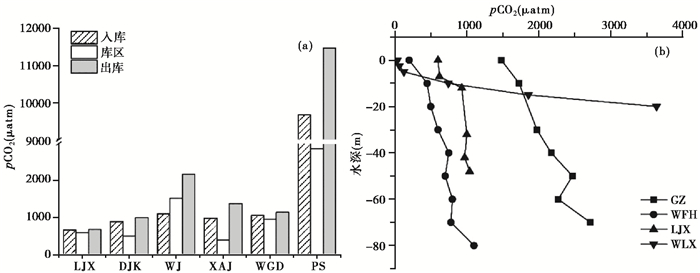
筑坝将异养的自然河流转变成自养的水库,改变了河流的连续性和水文情势:河流流速减缓,水体滞留时间延长,水深增加,浊度降低,库区水体表层浮游植物光合作用增强,导致库区水体pCO2下降(图 3a)[44];出库水体以pCO2较高的底层水为主,pCO2通常较大[19, 59];多数水库水体pCO2表现出出库>入库>库中的空间分布差异(图 3a)。乌江流域水库pCO2出库>库中>入库,可能与乌江为山区河流,其支流流速大、湍流强,导致入库水pCO2较小相关[65]。此外,水库pCO2还表现出随水深增加而增大的特点(图 3b)。这与太阳辐射随水深减少,生物光合作用减弱,而有机质的沉降和微生物呼吸作用随深度增强,释放CO2增多有关[44, 46, 51]。
|
图 3 水库不同位置pCO2的变化 图 3a:LJX——刘家峡水库(LiuJiaXia Reservoir),DJK——丹江口水库(DanJiangKou Reservoir),WJ——乌江流域多个水库均值(Reservoirs in WuJiang)[30],XAJ——新安江水库(XinAnJiang Reservoir),WGD——王圪堵水库(WangGeDu Reservoir),PS——Petit-Saut Reservoir;图 3b:GZ——光照水库(GuangZhao Reservoir),WFH——万峰湖水库(WanFengHu Reservoir),WLX——五里峡水库(WuLiXia Reservoir) Fig. 3 Spatial variation of pCO2 in the reservoirs |
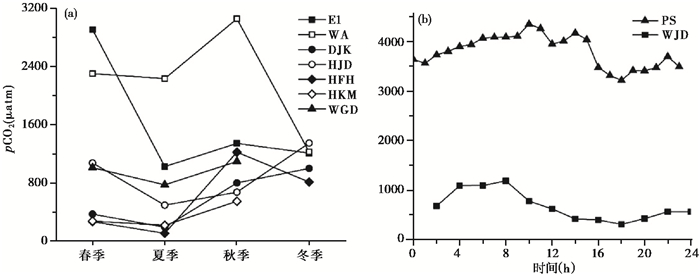
水库pCO2季节变化一般表现为秋冬季高,春夏季低(图 4a)的特点。结冰的水库Eastmain 1(E1)由于表面冰层的阻隔,冬季水体pCO2降低;春季,冰面消融导致pCO2急剧升高[22]。赣南万安水库(WA)冬季由于水体浊度远低于其他季节,其表层水体光合作用较强,特别是在中心库区,溶解CO2被大量吸收,导致水库表层pCO2较低[47]。
|
图 4 水库pCO2时间变化 图 4a:E1—Eastmain 1 Reservoir, WA—万家水库(WanAn Reservoir),DJK——丹江口水库(DanJiangKou Reservoir),HJD——洪家渡水库(HongJiaDu Reservoir),HFH——红枫湖水库(HongFengHu Reservoir),HKM——河口庙水库(HeKouMiao Reservoir),WGD——王圪堵水库(WangGeDu Reservoir);图 4b:PS——Petit-Saut Reservoir,WJD——乌江渡水库(WuJiangDu Reservoir) Fig. 4 Temporal variation of pCO2 in the reservoirs |
夏季浮游植物光合作用增强,消耗CO2,降低pCO2,部分水库pCO2可低于大气值,成为大气CO2的汇[24, 66]。此外,水库pCO2的季节变化与降雨分布有关,部分水库丰水期pCO2较高,枯水期较低,这是降雨稀释水库中DIC和Alk的结果[2, 20, 53];但强降雨事件带来的有机物也可导致pCO2升高,例如巴西湖泊在强降雨后,高饱和CO2的地下水输入使湖泊水体CO2增加,导致pCO2增加约10倍[67]。显然pCO2的季节变化显著受外源有机质季节性输入和矿化作用制约[20, 53, 67~68]。
水库pCO2日变化一般较小(图 4b),大多呈夜晚高、白天低趋势。这与温度、光照和水生植物光合作用日变化有关[35, 43, 48]。
4 水库pCO2变化的制约因素影响水库pCO2变化的主要因素有水温、pH、生物活动、外来水体的性质等。这些因素的不确定性增加了水库pCO2观测及计算难度。
4.1 水库水温和pH水库水温和pH值是影响水体pCO2的重要因素。pCO2与水体温度和pH均呈极显著负相关(表 2)。水库水体温度的时空变化引起水流运动模式和水生生物活动发生改变,导致水体物质的迁移模式和分解速率改变,进而影响pCO2[46]。水温的季节垂直分层变化,使水库上下层水体在化学条件和生物活性上有明显不同:夏季水库表层水温较高,水体热分层现象明显,水体中溶解气体和营养盐的上下交换受到抑制,影响水体碳氮的迁移、转化,微生物呼吸作用产生的CO2、CH4和氧化亚氮积累于库底;夏季结束后,水体上下层混合作用增强,库底积累的CH4气体在向上扩散过程中随水体溶解氧(DO)浓度增大被氧化为CO2[31],改变垂向pCO2分布。水温升高可增加气体分子的扩散速度,降低CO2的溶解度,导致水库pCO2降低,反之pCO2增高[55]。此外,水温变化可影响浮游植物的生长情势和微生物活性,进而影响pCO2[53]。水库表层水体温度对太阳辐射变化反应敏感,使得水体表层理化过程和生物过程变化较快,表层水体pCO2随水温变化较下层水体快[46, 52]。
| 表 2 pCO2与影响因子的相关性 Table 2 Correlation between pCO2 and influencing factors |
水体pH主要通过改变DIC的迁移-转化过程和水生生物代谢活动来影响pCO2[69]。水体不同pH值,DIC的存在形态不同,pH>9.4时,以CO32-为主,7.0 < pH < 8.3时,HCO3-占优势,pH < 6.4时,以CO2和H2CO3为主[70]。根据公式(3)~(5),水体pH值,尤其是低pH(5左右),对pCO2的计算结果影响较大[55]。这是两种效应耦合的结果[64]:1)水体DOC的有机酸阴离子可影响Alk;2)pH低则碳酸盐系统缓冲能力弱,导致计算pCO2对Alk更敏感。
4.2 生物活动水库水生生物的新陈代谢过程(公式6)可影响水体pCO2[69]:
|
(6) |
公式(6)中,P表示光合作用,R表示呼吸作用。水生生物光合作用消耗水中CO2,形成DO和浮游植物有机体,导致pCO2降低;呼吸作用反之[20, 46~47]。水体DO主要源自大气输入和浮游植物光合作用,浮游植物的呼吸作用和水体有机质的分解需要消耗DO[46]。水体中有机物分解的途径、产物受制于水体DO含量及其分布,并影响水体生源要素的循环[31]。水库水动力条件减弱,导致库区表层水体透明度增加,浮游植物光合作用增强,增加水体DO含量;受沉降作用影响,下层水体及沉积物表层有机质分解作用较强,消耗水体中DO并增加pCO2[46~47]。
研究显示一般营养水平的水库,其水体pCO2受到碳酸盐岩的风化和藻类等生物活动的共同控制,而极富营养化的水库,pCO2主要受到藻类等生物活动的控制[49]。浮游植物的产量常用叶绿素a(Chl-a)含量表征,故pCO2与DO、Chl-a含量呈显著负相关(表 2)。此外,太阳辐射、湖泊营养状况和水温等环境因子对湖泊/水库pCO2和CO2动态变化也有重要影响[71]。随着水体深度的增加,太阳辐射减弱,光合作用减弱,有机质分解作用加强,使得水中无机碳平衡逐渐受呼吸作用控制,故水体pCO2随着水体深度的增加逐渐升高[46~47, 52]。大量研究表明水库水体pCO2季节变化和昼夜变化主要受控于水库水体内部光合作用与呼吸作用平衡[47~48, 55]。这与多数湖泊[71~72]和河溪[73~74]水体的研究结果一致。
4.3 外来水的性质水库上游及周边来水的性质在一定程度上影响水库pCO2。降雨一方面稀释水体导致DIC和Alk降低,降低水体pCO2;另一方面将大量上游有机物质带入水库,使其在生物活动下产生大量CO2导致pCO2升高,这也是热带水库雨季pCO2较高的主要原因[20]。地下水渗入是河流湖泊DIC的主要来源之一[20, 74],CO2过饱和的地下水渗入可引起水库水体CO2剧增,进而增加水体pCO2[67]。
水库上游流域的岩性也在很大程度上影响着水库pCO2,与硅酸盐岩流域比较,碳酸盐岩流域内河流水体的pH、总碱度、电导率和DIC含量相对较高,通常有较高的pCO2[6, 51, 74]。如喀斯特典型发育区的贵州省兴义市万峰湖水库,周边及上游水体携带的有机质大量在库区沉积、分解并释放CO2,致使库区pCO2较高[51]。高氮的上游来水促进水库浮游植物光合作用,增大DO浓度,降低水库pCO2;有机碳含量高的上游水输入有利于增强水库细菌的呼吸作用,减少DO,增大CO2浓度,从而增大pCO2[53]。故水库水体pCO2常与DOC浓度呈极显著负相关与Alk呈极显著正相关(表 2)。此外,Wang等[75]研究表明,上游来水物质组成不同,水库pCO2差异明显,城市区域pCO2平均值(8204 μatm)>农业区域(1841 μatm)>森林区域(1374 μatm)。集水区营养物质的时空差异控制流域生态系统光合作用和呼吸作用的变化,进而制约pCO2[2, 53]。外源DOC矿化对寒温带湖泊水体表层pCO2的贡献可达30 % ~40 % [76]。
5 结语与展望目前,陆地水域碳的存在形式和动力学过程方面已有较多的研究,但是对于动态的水体pCO2定量研究仍处于初步监测阶段和计算阶段,很多过程和控制因素并不十分清楚。由于陆地水域的复杂性,特别是水文情势受人为剧烈干扰的水库,水体DIC含量变化频繁,导致pCO2时空波动较大,其研究的不确定性很大。为精确估算陆地水域碳储量,明确陆地水域碳动态在全球碳循环中的意义,建议加强以下研究:
(1) 提高陆地水体pCO2数据的准确性和陆地水-气界面CO2交换通量估算精度。水库水体pCO2受制于水体温度、pH、浮游植物生长、DO、DOC和DIC含量等因素,应加强对这些因素的监测;鉴于陆地水体DIC的监测结果与模型计算结果差异较大,影响定量估算陆地水域对地球碳循环的贡献[65],故完善陆地水体DIC模型应引起关注。
(2) 确定水-气界面CO2交换的pCO2临界值范围。水库在提供清洁能源的同时,其大气CO2的源/汇问题一直备受关注。水库/湖泊水体pCO2高于大气,但是不一定会向大气排放CO2。这是因为:1)水库水流速较低[77];2)水库水体pCO2需要大于水-气界面CO2交换的临界值才可能向大气释放CO2[78]。目前促使水-气界面CO2交换的pCO2临界值尚属未知。因此,确定水库水体pCO2临界值非常重要。
(3) 探究筑坝对河流DIC生物地球化学行为的改变机制。陆地水域DIC动力学受流域岩性、植被、水文情势和水生生物等多因素耦合控制。水文学家明晰水体水文情势,量化水资源及其滞留时间;生态学家深刻分析影响及改变DIC行为的生物化学机制,揭示陆地水域DIC时空变化特点及变化过程。水文学、湖沼学、生态学和地球化学等多学科研究群体共同努力,采取区域尺度系统观察方法,方可准确量化陆地水域DIC对地球系统C循环的贡献。
致谢: 感谢审稿专家和编辑部杨美芳老师提出宝贵的修改意见,在此一并致谢!
| [1] |
袁道先. 地球系统的碳循环和资源环境效应[J]. 第四纪研究, 2001, 21(3): 223-232. Yuan Daoxian. Carbon cycle in Earth system and its effects on environment and resources[J]. Quaternary Sciences, 2001, 21(3): 223-232. DOI:10.3321/j.issn:1001-7410.2001.03.004 |
| [2] |
Liu S, Lu X X, Xia X, et al. Dynamic biogeochemical controls on river pCO2 and recent changes under aggravating river impoundment:An example of the subtropical Yangtze River[J]. Global Biogeochemical Cycles, 2016, 30(6): 880-897. DOI:10.1002/2016GB005388 |
| [3] |
Raymond P A, Hartmann J, Lauerwald R, et al. Global carbon dioxide emissions from inland waters[J]. Nature, 2013, 503(7476): 355-359. DOI:10.1038/nature12760 |
| [4] |
Tranvik L J, Downing J A, Cotner J B, et al. Lakes and reservoirs as regulators of carbon cycling and climate[J]. Limnology and Oceanography, 2009, 54(6): 2298-2314. |
| [5] |
Aufdenkampe A K, Mayorga E, Raymond P A, et al. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere[J]. Frontiers in Ecology and the Environment, 2011, 9(1): 53-60. DOI:10.1890/100014 |
| [6] |
Han Q, Wang B, Wang F, et al. Carbon biogeochemical cycle is enhanced by damming in a karst river[J]. Science of the Total Environment, 2018, 616: 1181-1189. DOI:10.1016/j.scitotenv.2017.10.202 |
| [7] |
Demarty M, Bastien J. GHG emissions from hydroelectric reservoirs in tropical and equatorial regions:Review of 20 years of CH4 emission measurements[J]. Energy Policy, 2011, 39(7): 4197-4206. DOI:10.1016/j.enpol.2011.04.033 |
| [8] |
Roland F, Vidal L O, Pacheco F S, et al. Variability of carbon dioxide flux from tropical(Cerrado)hydroelectric reservoirs[J]. Aquatic Sciences, 2010, 72(3): 283-293. DOI:10.1007/s00027-010-0140-0 |
| [9] |
Fearnside P M. Greenhouse gas emissions from hydroelectric dams:controversies provide a springboard for rethinking a supposedly 'clean' energy source[J]. Climatic Change, 2004, 66(1-2): 1-8. DOI:10.1023/B:CLIM.0000043174.02841.23 |
| [10] |
Rosa L P, Santos M, Matvienko B, et al. Greenhouse gas emissions from hydroelectric reservoirs in tropical regions[J]. Climatic Change, 2004, 66(1-2): 9-21. DOI:10.1023/B:CLIM.0000043158.52222.ee |
| [11] |
Wang W, Roulet N T, Kim Y, et al. Modelling CO2 emissions from water surface of a boreal hydroelectric reservoir[J]. Science of the Total Environment, 2018, 612: 392-404. DOI:10.1016/j.scitotenv.2017.08.203 |
| [12] |
Barros N, Cole J J, Tranvik L J, et al. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude[J]. Nature Geoscience, 2011, 4(9): 593-596. DOI:10.1038/ngeo1211 |
| [13] |
Tremblay A, Lambert M, Gagnon L. Do hydroelectric reservoirs emit greenhouse gases?[J]. Environmental Management, 2004, 33: S509-S517. DOI:10.1007/s00267-003-9158-6 |
| [14] |
Prairie Y T, Alm J, Beaulieu J, et al. Greenhouse gas emissions from freshwater reservoirs:What does the atmosphere see?[J]. Ecosystems, 2018, 21(5): 1058-1071. DOI:10.1007/s10021-017-0198-9 |
| [15] |
Deemer B R, Harrison J A, Li S, et al. Greenhouse gas emissions from reservoir water surfaces:A new global synthesis[J]. BioScience, 2016, 66(11): 949-964. DOI:10.1093/biosci/biw117 |
| [16] |
Li S, Bush R T, Santos I R, et al. Large greenhouse gases emissions from China's lakes and reservoirs[J]. Water Research, 2018, 147: 13-24. DOI:10.1016/j.watres.2018.09.053 |
| [17] |
杨玉雪, 向鹏, 卢玮琦, 等. 贵州乌江渡水库沉积速率及碳氮埋藏通量估算[J]. 地球与环境, 2017, 45(1): 66-73. Yang Yuxue, Xiang Peng, Lu Weiqi, et al. The sedimentation rate and burial fluxes of carbon and nitrogen in Wujiangdu Reservoir, Guizhou, China[J]. Earth and Environment, 2017, 45(1): 66-73. |
| [18] |
Abril G, Guérin F, Richard S, et al. Carbon dioxide and methane emissions and the carbon budget of a 10-year old tropical reservoir(Petit Saut, French Guiana)[J]. Global Biogeochemical Cycles, 2005, 19(4): GB4007. DOI:10.1029/2005GB002457 |
| [19] |
喻元秀, 刘丛强, 汪福顺, 等. 洪家渡水库溶解二氧化碳分压的时空分布特征及其扩散通量[J]. 生态学杂志, 2008, 27(7): 1193-1199. Yu Yuanxiu, Liu Congqiang, Wang Fushun, et al. Spatiotemporal characteristics and diffusion flux of partial pressure of dissolved carbon dioxide in Hong Jiadu reservoir[J]. Chinese Journal of Ecology, 2008, 27(7): 1193-1199. |
| [20] |
Macklin P A, Suryaputra I G N A, Maher D T, et al. Carbon dioxide dynamics in a lake and a reservoir on a tropical island(Bali, Indonesia)[J]. PLoS ONE, 2018, 13(7): e0200948. DOI:10.1371/journal.pone.0198678 |
| [21] |
Wen Z, Song K, Shang Y, et al. Carbon dioxide emissions from lakes and reservoirs of China:A regional estimate based on the calculated pCO2[J]. Atmospheric Environment, 2017, 170: 71-81. DOI:10.1016/j.atmosenv.2017.09.032 |
| [22] |
Demarty M, Tremblay A. Long term follow-up of pCO2, pCH4 and emissions from Eastmain 1 boreal reservoir, and the Rupert diversion bays, Canada[J]. Ecohydrology and Hydrobiology, 2017. DOI:10.1016/j.ecohyd.2017.09.001.inpress |
| [23] |
Soumis N, Duchemin É, Canuel R, et al. Greenhouse gas emissions from reservoirs of the western United States[J]. Global Biogeochemical Cycles, 2004, 18(3): GB3022. DOI:10.1029/2003GB002197 |
| [24] |
Ran L, Li L, Tian M, et al. Riverine CO2 emissions in the Wuding River catchment on the Loess Plateau:Environmental controls and dam impoundment impact[J]. Journal of Geophysical Research:Biogeosciences, 2017, 122(6): 1439-1455. DOI:10.1002/2016JG003713 |
| [25] |
Chen H, Wu Y, Yuan X, et al. Methane emissions from newly created marshes in the drawdown area of the Three Gorges Reservoir[J]. Journal of Geophysical Research:Atmospheres, 2009, 114: D18301. DOI:10.1029/2009JD012410 |
| [26] |
Alin S R, Rasera M D F F, Salimon C I, et al. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets[J]. Journal of Geophysical Research, 2011, 116: G01009. DOI:10.1029/2010JG001398 |
| [27] |
梅航远, 汪福顺, 姚臣谌, 等. 万安水库春季二氧化碳分压的分布规律研究[J]. 环境科学, 2011, 32(1): 58-63. Mei Hangyuan, Wang Fushun, Yao Chenchen, et al. Diffusion flux of partial pressure of dissolved carbon dioxide in Wan'an Reservoir in spring[J]. Environment Science, 2011, 32(1): 58-63. DOI:10.3969/j.issn.1673-9655.2011.01.016 |
| [28] | |
| [29] |
Regnier P, Friedlingstein P, Ciais P, et al. Anthropogenic perturbation of the carbon fluxes from land to ocean[J]. Nature Geoscience, 2013, 6(8): 597-607. DOI:10.1038/NGEO1830 |
| [30] |
彭希, 刘丛强, 王宝利, 等. 筑坝对喀斯特河流水体溶解性无机碳地球化学行为的影响[J]. 科学通报, 2014, 59(4-5): 366-373. Peng Xi, Liu Congqiang, Wang Baoli, et al. The impact of damming on geochemical behavior of dissolved inorganic carbon in a karst river[J]. Chinese Science Bulletin, 2014, 59(4-5): 366-373. |
| [31] |
程炳红, 郝庆菊, 江长胜. 水库温室气体排放及其影响因素研究进展[J]. 湿地科学, 2012, 10(1): 121-128. Cheng Binghong, Hao Qingju, Jiang Changsheng. Research progress on the emission of greenhouse gases from reservoir and its influence factors[J]. Wetland Science, 2012, 10(1): 121-128. DOI:10.3969/j.issn.1672-5948.2012.01.017 |
| [32] |
Nakayama T, Pelletier G J. Impact of global major reservoirs on carbon cycle changes by using an advanced eco-hydrologic and biogeochemical coupling model[J]. Ecological Modelling, 2018, 387: 172-186. DOI:10.1016/j.ecolmodel.2018.09.007 |
| [33] |
Yang L, Lu F, Zhou X, et al. Progress in the studies on the greenhouse gas emissions from reservoirs[J]. Acta Ecologica Sinica, 2014, 34(4): 204-212. DOI:10.1016/j.chnaes.2013.05.011 |
| [34] |
Delmas R, Galy-Lacaux C, Richard S. Emissions of greenhouse gases from the tropical hydroelectric reservoir of Petit Saut(French Guiana)compared with emissions from thermal alternatives[J]. Global Biogeochemical Cycles, 2001, 15(4): 993-1003. DOI:10.1029/2000GB001330 |
| [35] |
Abril G, Richard S, Guérin F. In situ measurements of dissolved gases(CO2 and CH4)in a wide range of concentrations in a tropical reservoir using an equilibrator[J]. Science of the Total Environment, 2006, 354(2-3): 246-251. DOI:10.1016/j.scitotenv.2004.12.051 |
| [36] |
Kemenes A, Forsberg B R, Melack J M. CO2 emissions from a tropical hydroelectric reservoir(Balbina, Brazil)[J]. Journal of Geophysical Research, 2011, 116: G03004. DOI:10.1029/2010JG001465 |
| [37] |
Guérin F, Abril G, Richard S, et al. Methane and carbon dioxide emissions from tropical reservoirs:Significance of downstream rivers[J]. Geophysical Research Letters, 2006, 33(21): L21407. DOI:10.1029/2006GL027929 |
| [38] |
周梅, 叶丽菲, 张超, 等. 广东新丰江水库表层水体CO2分压及其影响因素[J]. 湖泊科学, 2018, 30(3): 770-781. Zhou Mei, Ye Lifei, Zhang Chao, et al. Partial pressure of carbon dioxide in the Xinfengjiang Reservoir of Guangdong Province and its influencing factors[J]. Journal of Lake Sciences, 2018, 30(3): 770-781. |
| [39] |
Crusius J, Wanninkhof R. Gas transfer velocities measured at low wind speed over a lake[J]. Limnology and Oceanography, 2003, 48(3): 1010-1017. DOI:10.4319/lo.2003.48.3.1010 |
| [40] |
Wanninkhof R. Relationship between wind speed and gas exchange[J]. Journal of Geophysical Resaech:Atmospheres, 1992, 12(6): 351-362. DOI:10.1029/92JC00188 |
| [41] |
Cole J J, Caraco N F. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6[J]. Limnology and Oceanography, 1998, 43(4): 647-656. DOI:10.4319/lo.1998.43.4.0647 |
| [42] |
Upstill-Goddard R, Watson A, Liss P, et al. Gas transfer velocities in lakes measured with SF6[J]. Tellus, Series B-Chemical and Physical Meteorology, 1990, 42(4): 364-377. DOI:10.3402/tellusb.v42i4.15230 |
| [43] |
Jin H, Yoon T K, Lee S H, et al. Enhanced greenhouse gas emission from exposed sediments along a hydroelectric reservoir during an extreme drought event[J]. Environmental Research Letters, 2016, 11(12): 124003. DOI:10.1088/1748-9326/11/12/124003 |
| [44] |
宫辰, 杨现坤, 田明扬, 等. 黄河源区水库二氧化碳逸出暖季变化规律及影响因素分析——以刘家峡水库为例[J]. 环境科学学报, 2018, 38. Gong Chen, Yang Xiankun, Tian Mingyang, et al. Variations of carbon dioxide evasion from reservoirs and its influencing factors in warm season in the headwater region of the Yellow River:A case study of the Liujiaxia Reservoir[J]. Acta Scientiae Circumstantiae, 2018, 38. |
| [45] |
张永领, 杨小林, 张东. 小浪底水库影响下的黄河花园口站和小浪底站pCO2特征及扩散通量[J]. 环境科学, 2015, 36(1): 40-48. Zhang Yongling, Yang Xiaolin, Zhang Dong. Partial pressure of carbon dioxide and carbon dioxide degassing fluxes of Huayuankou and Xiaolangdi Station affected by Xiaolangdi Reservoir[J]. Environmental Science, 2015, 36(1): 40-48. |
| [46] |
曹玉平, 袁热林, 焦树林, 等. 光照水库夏季分层期间二氧化碳分压分布特征[J]. 环境科学与技术, 2018, 41(6): 15-21. Cao Yuping, Yuan Relin, Jiao Shulin, et al. Distribution characteristics of partial pressure of carbon dioxide during thermal stratification in summer in Guangzhao Reservoir[J]. Environmental Science and Technology, 2018, 41(6): 15-21. |
| [47] |
汪福顺, 王宝利, 吴学谦, 等. 中国南方河道型水库CO2释放研究[J]. 矿物岩石地球化学通报, 2017, 36(1): 40-47. Wang Fushun, Wang Baoli, Wu Xueqian, et al. CO2 emission from the Wan'an Reservoir-Run-of-the-river reservoir in Southern China[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2017, 36(1): 40-47. DOI:10.3969/j.issn.1007-2802.2017.01.005 |
| [48] |
Peng X, Wang B, Liu C, et al. Diurnal variations of pCO2 in relation to environmental factors in the cascade reservoirs along the Wujiang River, China[J]. Chinese Journal of Geochemistry, 2012, 31(1): 41-47. DOI:10.1007/s11631-012-0547-5 |
| [49] |
彭希, 刘丛强, 王宝利, 等. 河流-水库体系水体表层pCO2特征及其扩散通量——以六冲河、洪家渡水库、红枫湖为例[J]. 地球与环境, 2013, 41(23): 97-103. Peng Xi, Liu Congqiang, Wang Baoli, et al. Spatiotemporal characteristics and diffusion flux of partial pressure of dissolved carbon dioxide in the river-reservoir system as exemplified by the Liuchonghe River, Hongjiadu Reservoir and Hongfenghu Lake[J]. Earth and Environment, 2013, 41(23): 97-103. |
| [50] |
Wang F, Wang B, Liu C, et al. Carbon dioxide emission from surface water in cascade reservoirs-river system on the Maotiao River, Southwest of China[J]. Atmospheric Environment, 2011, 45(23): 3827-3834. DOI:10.1016/j.atmosenv.2011.04.014 |
| [51] |
张倩, 焦树林, 梁虹, 等. 喀斯特地区水库回水区夏季水体二氧化碳分压变化特征及交换通量研究[J]. 水文, 2018, 38(1): 28-34. Zhang Qian, Jiao Shulin, Liang Hong, et al. Partial pressure variation and diffusion flux of carbon dioxide in reservoir backwater area in karast zone in summer[J]. Journal of China Hydrology, 2018, 38(1): 28-34. DOI:10.3969/j.issn.1000-0852.2018.01.005 |
| [52] |
刘文, 蒲俊兵, 于奭, 等. 广西五里峡水库夏季溶解无机碳行为的初步研究[J]. 环境科学, 2014, 35(8): 2959-2966. Liu Wen, Pu Junbing, Yu Shi, et al. Preliminary research on the feature of dissolved inorganic carbon in Wulixia Reservoir in summer, Guangxi, China[J]. Environmental Science, 2014, 35(8): 2959-2966. |
| [53] |
Li S, Zhang Q. Partial pressure of CO2 and CO2 emission in a monsoon-driven hydroelectric reservoir(Danjiangkou Reservoir), China[J]. Ecological Engineering, 2014, 71: 401-414. DOI:10.1016/j.ecoleng.2014.07.014 |
| [54] |
姚臣谌, 汪福顺, 吴以赢, 等. 新安江水库水体春季二氧化碳分压的分布规律研究[J]. 地球环境学报, 2010, 1(2): 150-156. Yao Chenchen, Wang Fushun, Wu Yiying, et al. The vernal distribution of dissolved carbon dioxide(pCO2) in the Xin'anjiang Reservoir[J]. Journal of Earth Environment, 2010, 1(2): 150-156. |
| [55] |
Chung S, Park H, Yoo J. Variability of pCO2 in surface waters and development of prediction model[J]. Science of the Total Environment, 2018, 622: 1109-1117. |
| [56] |
Jin H, Yoon T K, Begum M S, et al. Longitudinal discontinuities in riverine greenhouse gas dynamics generated by dams and urban wastewater[J]. Biogeosciences, 2018, 15(20): 6349-6369. DOI:10.5194/bg-15-6349-2018 |
| [57] |
Varol M. CO2 emissions from hydroelectric reservoirs in the Tigris River basin, a semi-arid region of Southeastern Turkey[J]. Journal of Hydrology, 2019, 569: 782-794. DOI:10.1016/j.jhydrol.2019.01.002 |
| [58] |
Bergstrom A K, Algesten G, Sobek S, et al. Emission of CO2 from hydroelectric reservoirs in northern Sweden[J]. Archiv fur Hydrobiologie, 2004, 159(1): 25-42. DOI:10.1127/0003-9136/2004/0159-0025 |
| [59] |
Guérin F, Abril G L, Ser A D, et al. Gas transfer velocities of CO2 and CH4 in a tropical reservoir and its river downstream[J]. Journal of Marine System, 2007, 66(1-4): 161-172. DOI:10.1016/j.jmarsys.2006.03.019 |
| [60] |
Curtarelli M P, Ogashawara I, de Araújo C A S, et al. Carbon dioxide emissions from Tucuruí Reservoir(Amazon biome):New findings based on three-dimensional ecological model simulations[J]. Science of the Total Environment, 2016, 551-552: 676-694. DOI:10.1016/j.scitotenv.2016.02.001 |
| [61] |
Yoon T K, Jin H, Oh N H, et al. Technical note:Assessing gas equilibration systems for continuous pCO2 measurements in inland waters[J]. Biogeosciences, 2016, 13(13): 3915-3930. DOI:10.5194/bg-13-3915-2016 |
| [62] |
Kling G W, Kipphut G W, Miller M C. The flux of CO2 and CH4 from lakes and rivers in arctic Alaska[J]. Hydrobiologia, 1992, 240(1-3): 23-26. DOI:10.1007/BF00013449 |
| [63] |
Hunt C W, Salisbury J E, Vandemark D. Contribution of non-carbonate anions to total alkalinity and overestimation of pCO2 in New England and New Brunswick rivers[J]. Biogeosciences, 2011, 8(10): 3069-3076. DOI:10.5194/bg-8-3069-2011 |
| [64] |
Abril G, Bouillon S, Darchambeau F, et al. Technical Note:Large overestimation of pCO2 calculated from pH and alkalinity in acidic, organic-rich freshwaters[J]. Biogeosciences, 2015, 12(1): 67-78. DOI:10.5194/bg-12-67-2015 |
| [65] |
Duvert C, Butman D E, Marx A, et al. CO2 evasion along streams driven by groundwater inputs and geomorphic controls[J]. Nature Geoscience, 2018, 11(11): 813-818. DOI:10.1038/s41561-018-0245-y |
| [66] |
Wang F, Cao M, Wang B, et al. Seasonal variation of CO2 diffusion flux from a large subtropical reservoir in East China[J]. Atmospheric Environment, 2015, 103: 129-137. DOI:10.1016/j.atmosenv.2014.12.042 |
| [67] |
Marotta H, Duarte C M, Pinho L, et al. Rainfall leads to increased pCO2 in Brazilian coastal lakes[J]. Biogeosciences, 2010, 7(5): 1607-1614. DOI:10.5194/bg-7-1607-2010 |
| [68] |
Jonsson A, åberg J, Jansson M. Variations in pCO2 during summer in the surface water of an unproductive lake in Northern Sweden[J]. Tellus, Series B-Chemical and Physical Meteorology, 2007, 59(5): 797-803. DOI:10.1111/j.1600-0889.2007.00307.x |
| [69] |
戴树桂. 环境化学[M]. 北京: 高等教育出版社, 2006: 145-159. Dai Shugui. Environmental Chemistry[M]. Beijing: Higher Education Press, 2006: 145-159. |
| [70] |
Golterman H L, Clymo R S, Ohnstad M A M. Methods for Physical and Chemical Analysis of Fresh Waters[M]. Oxford: Blackwell Scientific Blackwell Scientific, 1978: 337-338.
|
| [71] |
Yang R, Xu Z, Liu S, et al. Daily pCO2 and CO2 flux variations in a subtropical mesotrophic shallow lake[J]. Water Research, 2019, 153: 29-38. DOI:10.1016/j.watres.2019.01.012 |
| [72] |
王仕禄, 万国江, 刘丛强, 等. 云贵高原湖泊CO2的地球化学变化及其大气CO2源汇效应[J]. 第四纪研究, 2003, 23(5): 581. Wang Shilu, Wan Guojiang, Liu Congqiang, et al. Geochemical changes of its effects on source or fates of CO2 in the lakes of Yunnan-Guizhou Plateau[J]. Quaternary Sciences, 2003, 23(5): 581. DOI:10.3321/j.issn:1001-7410.2003.05.014 |
| [73] |
莫雪, 蒲俊兵, 袁道先, 等. 亚热带典型岩溶区地表溪流溶解无机碳昼夜变化特征及其影响因素[J]. 第四纪研究, 2014, 34(4): 873-880. Mo Xue, Pu Junbing, Yuan Daoxian, et al. Diel variation and influence factors of dissolved inorganic carbon in a surface creek fed by a karst subterranean stream in subtropical area, SW China[J]. Quaternary Sciences, 2014, 34(4): 873-880. DOI:10.3969/j.issn.1001-7410.2014.04.20 |
| [74] |
章程, 汪进良, 肖琼. 桂林潮田河溶解无机碳来源与昼夜动态变化[J]. 第四纪研究, 2017, 37(6): 1283-1292. Zhang Cheng, Wang Jinliang, Xiao Qiong. The sources and diurnal changes of dissolved inorganic carbon in Chaotian River, Gui Lin, China[J]. Quaternary Sciences, 2017, 37(6): 1283-1292. |
| [75] |
Wang X, He Y, Yuan X, et al. Greenhouse gases concentrations and fluxes from subtropical small reservoirs in relation with watershed urbanization[J]. Atmospheric Environment, 2017, 154: 225-235. DOI:10.1016/j.atmosenv.2017.01.047 |
| [76] |
Vachon D, Prairie Y T, Guillemette F, et al. Modeling allochthonous dissolved organic carbon mineralization under variable hydrologic regimes in boreal lakes[J]. Ecosystems, 2017, 20(4): 781-795. DOI:10.1007/s10021-016-0057-0 |
| [77] |
Deirmendjian L, Abril G. Carbon dioxide degassing at the groundwater-stream-atmosphere interface:Isotopic equilibration and hydrological mass balance in a sandy watershed[J]. Journal of Hydrology, 2018, 558: 129-143. DOI:10.1016/j.jhydrol.2018.01.003 |
| [78] |
Tromp-Van Meerveld H J, Mcdonnell J J. Threshold relations in subsurface stormflow:2. The fill and spill hypothesis[J]. Water Resources Research, 2006, 42(2): W02411. DOI:10.1029/2004WR003800 |
2 Southern Laboratory of Ocean Science and Engineering(Zhuhai), Zhuhai 519000, Guangdong;
3 Guangdong Provincial Key Laboratory of Mineral Resource & Geological Processes, Guangzhou 510275, Guangdong)
Abstract
Photosynthesis of assimilated carbon dioxide (CO2) and microbial respiration processes simultaneously occur within the natural waters. CO2 exchange between water bodies and the atmosphere constitutes a major part of the global carbon cycle. The CO2 current direction and flux between water column and atmosphere are mainly controlled by CO2 partial pressure (pCO2) between the atmosphere and the surface water bodies. The pCO2 values of water bodies can be obtained by on-site instrumental detection on the basis of gas composition change near the water surface layer or by empirical calculation in terms of measuring hydro-chemical parameters. Up to now, with regard to CO2 dynamics in inland waters, especially in reservoir water bodies formed by damming, the calculation method of hydro-chemical parameters is generally conducted to calculate the pCO2 values due to the complex spatiotemporal variability of atmospheric composition near water surface layer. It is reported that about 70.97% of pCO2 values in the global reservoirs are higher than the atmospheric pCO2. On the global scale, the surface water pCO2 in the reservoir shows a gradual decline from tropical zone to boreal zone; for a single reservoir, the pCO2 generally presents a variation pattern of "outlet of the reservoir > inlet of the reservoir > in the reservoir", and the pCO2 rises with increasing depth. The pCO2 temporal variation trend in the reservoirs surface water is generally winter > summer, snow melting period > freezing period, and night > day. The pCO2 of the reservoir water is the result of chemical elements equilibrium which varies in a complicated pattern, and is influenced by many factors, such as water temperature, water pH, aquatic biological activities and the mixing of exotic water. In order to accurately quantify the exchanged CO2 flux at the water-air interface, it is necessary for hydrologists, limnologists, ecologists and geochemists to cooperation to conduct field observations at reservoir watershed scale, to improve the calculation model of dissolved inorganic carbon in water, to explore the carbon dynamics mechanism of the reservoir water bodies, and to provide reliable data for studying global carbon cycle and predicting climate change. 2019, Vol.39
2019, Vol.39

