② 南方科技大学海洋科学与工程系, 深圳 518055;
③ 中国地质大学(北京)生物地质与环境地质国家重点实验室, 北京 100083;
④ 中国地质大学(武汉)生物地质与环境地质国家重点实验室, 武汉 430074)
古菌是生命三域之一[1],它们可能是地球上最古老的生命形式,广泛分布于湖泊、海洋、土壤、热泉等正常和极端环境中,在生物地球化学循环中扮演着重要的角色[2, 3]。类异戊二烯甘油醚类化合物是古菌细胞膜的重要成分[4, 5]。受外部生长环境的影响,古菌会调节自身所合成的这些细胞膜脂组成[6~8]。同时,由于不同古菌种属合成的细胞膜脂成分不同,不同环境中古菌群落结构的差异也会引起脂类组成变化[9~12]。因此,环境样品中的类异戊二烯甘油醚类化合物具有指示气候环境变化的潜力。
在有机地球化学领域,目前研究较为成熟普遍的古菌细胞膜脂是类异戊二烯甘油二烷基甘油四醚类化合物(Isoprenoid Glycerol Dialkyl Glycerol Tetraethers,简称isoGDGTs,图 1)[13]。这类大分子化合物结构相对稳定,不易降解,在古老的白垩纪和侏罗纪地质样品中都能被检出[14~17],是古环境研究理想的生物标志物。目前,研究者们已建立了一系列的基于isoGDGTs分布的古气候代用指标,如:定量重建水体古温度的TEX86指标(TetraEther indeX of tetraethers consisting of 86 carbon atoms)[18]、示踪天然气水合物降解影响的甲烷指数MI(Methane Index)[10]、衡量陆源有机质输入的BIT指标(Branched and Isoprenoid Tetraether index)[19]、恢复水体古盐度的ACE指标(Archaeol and Caldarchaeol Ecometric)[20]和指示干旱化的Ri/b指标(the Ratio of Isoprenoid vs. Branched GDGTs)[21],等等。通过对保存在地质体中相关甘油二烷基甘油四醚类化合物(Glycerol Dialkyl Glycerol Tetraethers,简称GDGTs)的分析,可以定性乃至定量重建过去区域和全球气候环境变化的历史[13, 22, 23]。

|
图 1 本研究讨论的isoGDGTs(GDGT-0、GDGT-1、GDGT-2、GDGT-3、crenarchaeol、crenarchaeol′)和OH-GDGTs(OH-GDGT-0、OH-GDGT-1、OH-GDGT-2) 的结构图 Fig. 1 Chemical structures of isoGDGTs(GDGT-0, GDGT-1, GDGT-2, GDGT-3, crenarchaeol, and crenarchaeol′) and OH-GDGTs(OH-GDGT-0, OH-GDGT-1, and OH-GDGT-2) discussed in the text |
最近,Liu等[24, 25]通过核磁共振和液相色谱-质谱分析,从海洋沉积物中检测鉴定出一系列GDGTs烷基链长上含有一个羟基的OH-GDGTs(monohydroxy-GDGTs)和含有两个羟基的2OH-GDGTs(dihydroxy-GDGTs)。其中,按照五元环的数目,OH-GDGTs可分为OH-GDGT-0、OH-GDGT-1和OH-GDGT-2(图 1)。它们在海洋沉积物样品中普遍存在且含量可观,在一些样品中占总GDGTs的比例可高达8 % [25];Huguet等[26]随后对全球不同地区海洋和湖泊沉积物中的OH-GDGTs做了调查,发现OH-GDGTs在高纬和低温水域含量很高,在海洋表层沉积物中其相对含量与温度有显著的负相关关系;Fietz等[27]、Kaiser和Arz[28]通过对大西洋北海和波罗的海的研究进一步证实OH-GDGTs的相对含量与温度负相关,并发现OH-GDGTs的环数分布(OH-GDGT-0/OH-GDGT-1) 也与温度有关;Lü等[29]对我国边缘海中OH-GDGTs的研究也表明OH-GDGTs的相对含量随纬度的升高而增加,同时,他们提出OH-GDGTs的环化指数(Ring Index)RI-OH或RI-OH′可作为重建过去海表面温度(SST)变化的新指标。
目前,尽管前人对海洋环境中的OH-GDGTs已做了很多工作[24~33],其他环境中OH-GDGTs的研究还很少[26]。位于青藏高原东北部的青海湖,是我国最大的封闭咸水湖(盐度14~16g/L;pH=8.8~9.3);受东亚季风、西南季风和西风的共同影响,该区域对全球气候和环境变化非常敏感[34~36],一直以来是第四纪古环境研究的热点地区[35~47]。本文报道了青海湖晚全新世沉积物中OH-GDGTs的检出,并讨论了OH-GDGTs的主要来源以及控制其含量变化的主要因素,以期为OH-GDGTs在湖泊古环境研究中的应用提供参考。
2 材料方法青海湖海拔约3193m,面积约4400km2,被大通山、日月山和青海南山所包围[48]。湖水平均深度约21m,最深处在湖南盆约27m[42]。该地区年平均气温约为1.2℃[49]。湖水在夏季分层(温跃层10~15m),上层水温12~15℃,下层水温约6℃[50, 51];在冬季会形成逆温层,每年冰冻期3~4个月(12月至来年3月),冰冻厚度可达0.8m[52]。湖区年平均降雨量约400mm,主要集中在夏季,6~8月份降水约占全年的65 % [43];蒸发量远大于降雨量,前者约为后者的3~4倍[39, 53]。青海湖入湖河流主要分布在北部和西北部,5条最大的河流布哈河、沙流河、哈尔盖河、泉吉河和黑马河各占总河流输入水量的51.4 %、16.1 %、15.9 %、3.6 %和0.7 % [49]。目前,地表径流和大气降水约各占外部输入水量的42 %,剩下的约16 %主要来自地下水入渗[49]。青海湖水体滞留时间约为33.4年[50],湖区平均风速约为4~6m/s[52]。由于人口稀少,青海湖地区受人类活动的影响很小[54]。
2011年8月,我们在东南湖盆水深约24m处采得一段长约5.8m的沉积岩芯QH-2011钻孔(36°39′34″N,100°35′37″E)。岩芯被现场切为17段(每段30~40cm)保存在干冰箱中运至实验室;随后,在无尘室中将每段样品按2cm间隔分样。该钻孔的GDGTs数据我们之前的研究已报道[55~57];钻孔深度年代模式为:0~315cm,年代(a B P.)=22.43×深度(cm);316~579cm,年代(a B P.)=40.68×深度(cm)-5762[55]。本研究只分析钻孔上部337cm的孔深、约8ka以来12个样品,样品的分辨率约为700年(表 1)。
| 表 1 本文所讨论的青海湖QH-2011钻孔样品OH-GDGT数据 Table 1 OH-GDGT data of core QH-2011 of Lake Qinghai discussed in this study |
样品经冷冻干燥并研磨后,以体积比为9︰1的甲醇︰二氯甲烷进行超声振荡萃取其中的有机组分3次。提取液合并后在氮气下吹干,用硅胶柱分离出非极性组分(正己烷洗脱)与极性组分(体积比1︰1的甲醇︰二氯甲烷洗脱)。含有GDGTs和OH-GDGTs的极性组分用体积比99︰1的正己烷︰异丙醇过0.22μm的PTFE滤膜后在高效液相色谱-大气压化学电离-质谱仪(LC-APCI-MS)上进行分析,仪器型号为岛津LCMS-8030三重四极杆液质联用仪。所用液相色谱柱为Inertsil SIL-100A硅胶柱(250mm×4.6mm,3μm),柱温40℃。流动相A相为正己烷,B相为异丙醇。洗脱程序为(B相比例):0~10分钟,3 %;10~30分钟,线性升至11 %;30~31分钟,线性升至100 %;31~38分钟,100 %;38~39分钟,线性降至3 %;39~52分钟,3 %。总流动相流速为0.8ml/分钟。APCI源温度350℃,脱溶剂管温度260℃。质谱方法采用选择离子扫描(SIM)模式检测特定质荷比(m/z)离子,包括isoGDGTs和OH-GDGTs相关的1302、1300、1298、1296和1292,以及支链GDGTs(brGDGTs)相关的1050、1048、1046、1036、1034、1032、1022、1020、1018等。
3 结果 3.1 OH-GDGTs的检出OH-GDGT-0、OH-GDGT-1、OH-GDGT-2质子化离子([M+H]+)的m/z分别为1318、1316和1314。然而,由于OH-GDGTs在APCI源中容易脱水形成[M+H-18]+离子,它们在m/z为1300、1298和1296的提取离子流色谱图(EIC)中也能够产生信号[24, 25];与isoGDGTs相比,OH-GDGTs中引入了羟基极性增强,因此在正相液相色谱条件下它们比isoGDGTs需要更长的时间才能洗脱出[25]。我们对青海湖钻孔沉积物进行GDGTs分析时发现,在m/z为1300、1298和1296的EIC图中,计算TEX86所用的GDGT-1、GDGT-2和GDGT-3出峰后会依次分别各出现一个含量较高的色谱峰(图 2,峰A~C)。同时这3个峰的洗脱时间也滞后于brGDGTs。这一出峰顺序与前人报道的OH-GDGTs的洗脱特征一致[24, 27]。因此,根据已发表的前人研究结果[24~27],3个峰A、B、C应分别对应于OH-GDGT-0、OH-GDGT-1和OH-GDGT-2。
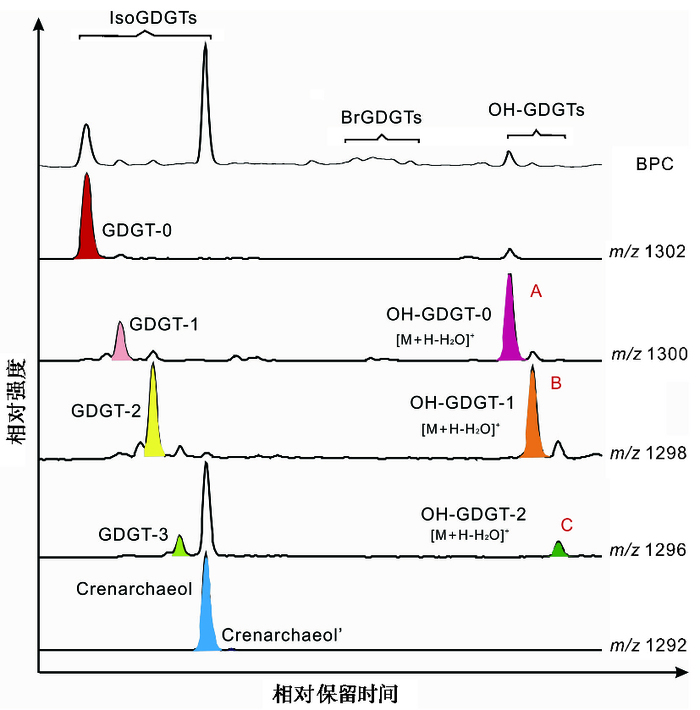
|
图 2 青海湖沉积物典型样品(B2-14) 的isoGDGTs和OH-GDGTs色谱图 Fig. 2 Example chromatograms of a Lake Qinghai sediment sample(B2-14) |
OH-GDGTs普遍存在于海洋沉积物中[25, 26]。我们按照青海湖钻孔样品同样的仪器方法对海洋样品进行分析时,在相同的保留时间也可检测到这3个峰。另外,在对青海湖沉积物进行全扫描分析时,A、B、C对应的峰在提取的m/z分别为1318、1316、1314的色谱图中也有显示。这些结果进一步证明A、B、C峰为OH-GDGTs的信号。
3.2 OH-GDGTs的含量和分布本研究中OH-GDGTs的[M+H]+(1318、1316、1314) 峰强度远低于对应[M+H-18]+(1300、1298、1296) 的峰强度,表明本实验条件下OH-GDGTs主要是以[M+H-18]+离子形式存在的,这与Liu等[25]的结果一致。因此,我们仅通过分析m/z为1300、1298和1296的EIC结果对OH-GDGT-0、OH-GDGT-1和OH-GDGT-2对应色谱峰进行峰面积的积分和定量(假设它们与C46GDGT标准具有相同的响应因子);另外,根据m/z为1302、1300、1298、1296和1292的EIC谱图中GDGT-0、GDGT-1、GDGT-2、GDGT-3和crenarchaeol(crenarchaeol′)的对应峰面积对isoGDGTs进行定量(图 2)。
除B5-32样品OH-GDGT-1和OH-GDGT-2低于检测限外,本研究分析的其他青海湖中晚全新世沉积物样品中均可检出OH-GDGT-0、OH-GDGT-1和OH-GDGT-2。样品中的OH-GDGTs以OH-GDGT-0为主,OH-GDGT-1次之,OH-GDGT-2最少(见表 1),其中,OH-GDGT-0含量变化范围为0.8~41.7ng/g,OH-GDGT-1含量变化范围为0.0~9.4ng/g,OH-GDGT-2含量变化范围为0.0~2.4ng/g;各OH-GDGT的含量在中全新世早期较低,4.8ka左右最高,在晚全新世基本维持在较高水平(图 3a)。
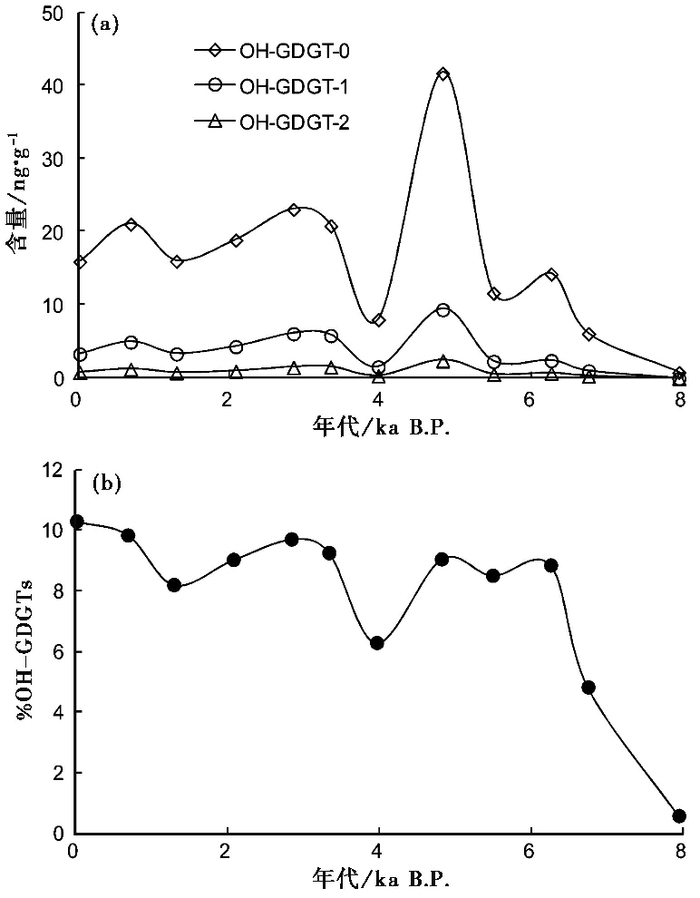
|
图 3 青海湖QH-2011钻孔8ka以来OH-GDGTs含量(a)和相对含量(b)的变化 Fig. 3 Variations in the concentrations of OH-GDGTs (a) and the relative abundance of OH-GDGTs (b) in core QH-2011 of Lake Qinghai for the past 8ka |
总OH-GDGTs含量变化范围为0. 8~53. 6ng/g,平均值为22. 0ng/g,比总isoGDGTs的含量(142. 7~539. 5ng/g,平均值为246. 9ng/g)系统偏低(见表 1)。参照Fietz等[27]的计算方法,我们用%OH-GDGTs来表示OH-GDGTs的百分含量:
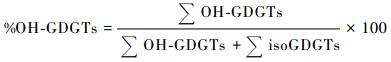
|
%OH-GDGTs值变化范围为0.56~10.28,在中全新世早期较低,之后快速升高并保持在6~10之间(见表 1和图 3b),表现出与各OH-GDGT含量类似的变化特征。
4 讨论 4.1 青海湖沉积物OH-GDGTs的来源目前的研究表明,奇古菌[58~61]和广古菌[24, 25]都能够产生OH-GDGTs。对青海湖钻孔样品的分析显示(图 4),OH-GDGTs相对含量(fOH-GDGTs)与crenarchaeol相对含量(fcrenarchaeol)之间具有很好的正相关关系(R2=0.93;p < 0.01),而与GDGT-0相对含量(fGDGT-0)负相关(R2=0.96;p < 0.01)。Crenarchaeol是奇古菌的特征标记物[62~64],而广古菌能合成GDGT-0但不能合成crenarchaeol[13, 65~66]。因此,青海湖钻孔样品中OH-GDGTs与crenarchaeol的正相关关系可能表明青海湖沉积物中所检出的OH-GDGTs主要来自奇古菌;另外,QH-2011钻孔中OH-GDGTs含量变化与氨氧化奇古菌amoA基因丰度[67]变化基本一致(图 5),进一步表明青海湖中的OH-GDGTs可能主要来自奇古菌。
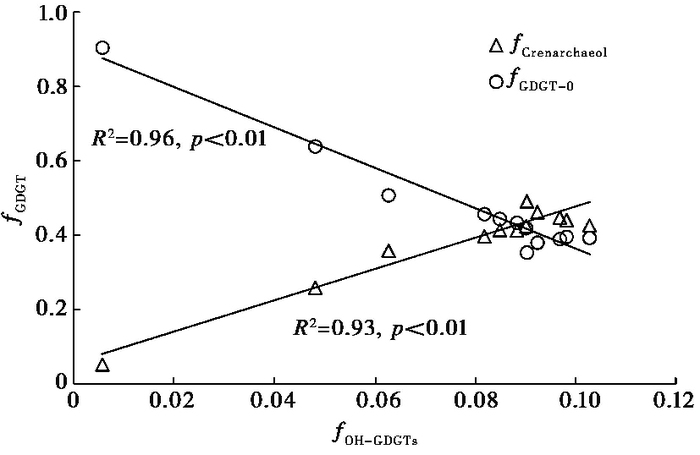
|
图 4 OH-GDGTs相对含量(fOH-GDGTs)与GDGT相对含量(fGDGT)的关系 三角表示fOH-GDGTs与fcrenarchaeol的关系,圆圈表示fOH-GDGTs与fGDGT-0的关系 Fig. 4 Correlations between the relative abundance of OH-GDGTs(fOH-GDGTs) and the relative abundance of two dominant GDGTs((fGDGT). Triangles denote the relationship between fOH-GDGTs and fcrenarchaeol, and circles denote the relationship between fOH-GDGTs and fGDGT-0 |
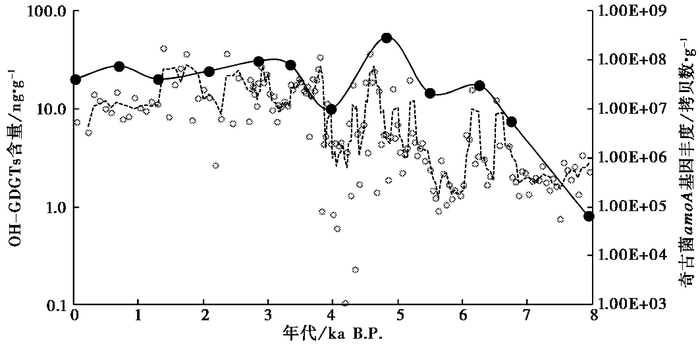
|
图 5 青海湖QH-2011钻孔8ka以来OH-GDGTs含量(对数坐标,实线+黑点)与奇古菌amoA基因丰度(对数坐标,三点平滑虚线+圆圈;数据来自Yang等[67])的变化 Fig. 5 Variations in the concentration of OH-GDGTs(the solid line and black dots)and thaumarchaeotal amoA gene abundance (the 3-point smoothing dotted line and circles, data from Yang et al.[67])in core QH-2011 of Lake Qinghai for the past 8ka |
土壤中也含有大量的奇古菌,且主要以group 1.1b奇古菌为主[68, 69]。然而,在奇古菌里,目前只在培养的group 1.1a类群中发现了OH-GDGTs[58~61],在group 1.1b培养物中尚没有OH-GDGTs存在的报道[11]。因此,在流域土壤奇古菌以group 1.1b为主的情况下,土壤OH-GDGTs的含量应该很低,不会对湖泊OH-GDGTs产生显著贡献。事实上,我们分析了几个青海湖周围土壤样品中的古菌四醚类化合物,未检测到明显的OH-GDGTs,尤其是OH-GDGT-1和OH-GDGT-2。所以我们推测青海湖中8ka以来钻孔沉积物中的OH-GDGTs可能主要来源于湖泊自生的group 1.1a奇古菌。然而,由于目前对不同环境和不同古菌种属OH-GDGTs的研究还比较少,尚不能完全排除其他古菌是青海湖中OH-GDGTs主要来源的可能性。
4.2 青海湖沉积物OH-GDGTs含量的控制因素目前,对于全球不同地区海洋表层沉积物的研究表明OH-GDGTs的相对含量随温度的降低而增加[26, 29]。最近Huguet等[70]通过分子动力学模拟,发现OH-GDGTs中羟基的引入有助于维持低温环境下的跨膜运输,这为OH-GDGTs的相对含量与温度的负相关关系提供了机理上的认识。然而,在青海湖中,中全新世早期以来%OH-GDGTs值增加了9.72(表 1和图 3),根据Lü等[29]最近得到的我国边缘海中OH-GDGTs相对含量与温度的关系,对应于温度下降了23℃,大大超过了青藏高原地区中晚全新世的温度变化范围(约4℃)[71]。因此,我们认为青海湖沉积物OH-GDGTs含量的变化可能主要受控于其他因素(尽管不排除温度的影响)。
研究表明,湖泊中的浮游奇古菌似乎更多的生活在次表层水体附近[72, 73]。这可能是由于以下两个因素:首先,奇古菌需要浮游生物来源的沉降微粒有机碳等释放的铵盐作为底物,但是在透光层铵盐可能会被更具竞争力的光合自养或者异养生物利用,所以生长缓慢的化能自养奇古菌主要生活在次表层水体,那里其他生物对铵盐的竞争较弱[13, 74];另外,也有报道指出强光对一些奇古菌具有抑制作用,这可能也与奇古菌较少生活在表层水中有关[13, 75]。因此,在湖泊较浅时,过浅的湖水无法提供水生奇古菌适宜的生存环境;而随着湖水深度的增加,水体出现分层,水生奇古菌含量才会显著增加,这样,湖水深度的变化可能会显著影响奇古菌的含量。
由于青海湖中的OH-GDGTs可能主要来源于水生奇古菌,而总isoGDGTs可由多种古菌普遍产生,我们进一步推断该湖中OH-GDGTs的产量和相对含量% OH-GDGTs也主要受水深控制。古湖岸线[76]、总有机碳同位素[77, 78]和crenarchaeol的百分含量(%cren)[55]是青海湖湖水深度变化的有效指标。在QH-2011钻孔沉积物中,OH-GDGTs的含量和相对含量的变化与古湖岸线、总有机碳同位素和%cren重建的湖泊水位变化具有类似的趋势(图 6),表明湖水深度对OH-GDGTs的含量和相对含量变化具有控制作用。
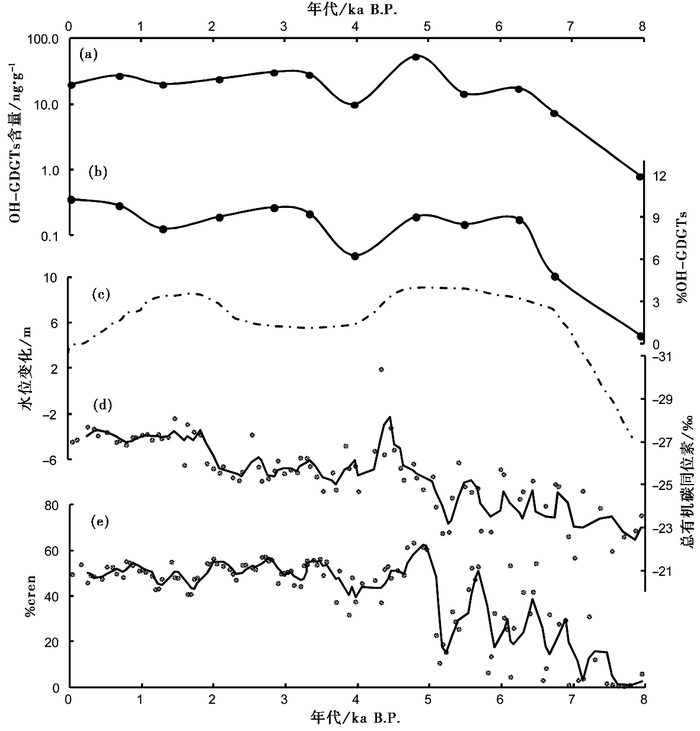
|
图 6 QH-2011钻孔中8ka以来各指标的变化 (a)OH-GDGTs含量(对数坐标);(b) % OH-GDGTs;(c)根据古湖岸线等重建的青海湖水位变化[76];(d)总有机碳同位素三点平滑曲线[55],偏负的值表明湖水较深[77];(e)crenarchaeol的百分含量(% cren)三点平滑曲线,% cren值越大表明湖水越深[55] Fig. 6 Changes in various proxies in core QH-2011 of Lake Qinghai for the past 8ka. (a)The concentration of OH-GDGTs; (b)The relative abundance of OH-GDGTs; (c)Reconstructed shoreline history[76]; (d)3-point moving total organic carbon isotope record[55], with more negative values indicating higher lake level[77]; (e)3-point moving % cren record, with higher values indicating higher lake level[55] |
海洋环境中的古菌群落较为单一[79],因而海洋沉积物中OH-GDGTs相对含量的变化可能反映了单一古菌种属细胞膜成分对温度的响应。相对于海洋环境,湖泊环境中其他水文条件的变化往往更大,古菌群落也较为复杂[80~82],这可能会严重影响%OH-GDGTs作为温度指标的可靠性。然而,另一方面,由于湖泊中OH-GDGTs可能主要来源于自生的group 1.1a奇古菌,而isoGDGTs可由多种古菌产生,湖泊沉积物中OH-GDGTs含量和相对含量的变化可能主要反映了水文条件变化导致的古菌群落的变化。青海湖中OH-GDGTs含量和相对含量变化与湖泊水位演化的一致性也表明,在古湖沼学研究中,沉积物中的OH-GDGTs可以用于指示湖水深度变化。
5 结论(1) 通过高效液相色谱-大气压化学电离-质谱分析,我们发现OH-GDGTs普遍存在于青海湖QH-2011钻孔8ka以来沉积物中,其总含量变化范围为0.8~53.6ng/g,平均值为22.0ng/g,这是我国湖泊中OH-GDGTs存在的首次报道。
(2) QH-2011钻孔8ka以来沉积物中OH-GDGTs相对含量与奇古菌特征标志物crenarchaeol相对含量之间具有很好的正相关关系(R2=0.93;p < 0.01),且OH-GDGTs含量变化与氨氧化奇古菌amoA基因丰度变化基本一致,表明OH-GDGTs可能主要来源于奇古菌。
(3) 由于青海湖湖水深度的变化可能会显著影响奇古菌的含量,而OH-GDGTs主要来源于奇古菌,我们推断青海湖中%OH-GDGTs的变化可能主要反映了湖水深度变化导致的古菌群落结构的变化。OH-GDGTs的含量及相对含量与古湖岸线、总有机碳同位素和%cren重建的湖泊水位具有类似的变化趋势,表明湖水深度对OH-GDGTs的产量具有控制作用,尽管不排除温度的影响。因此,古湖相沉积物中高丰度OH-GDGTs的检出可能指示了深水沉积环境,这与海洋环境中%OH-GDGTs主要反映温度的状况不同。未来的研究仍需进一步明确湖泊中OH-GDGTs的来源及其生理学意义和生态学作用。
致谢: 感谢中国地质大学(武汉)的杨渐博士提供了奇古菌16S rRNA基因丰度原始数据;感谢中国地质大学(北京)以及中国科学院南京地理与湖泊研究所的工作组在野外工作中给予的帮助。本研究得到国家自然科学基金项目(批准号:41573005) 和中国科学院“西部之光”人才培养引进计划项目(批准号:XAB2015B01) 共同资助。
| 1 |
Woese C R, Kandler O, Wheelis M L. Towards a natural system of organisms:Proposal for the domains Archaea, Bacteria, and Eucarya. Proceedings of the National Academy of Sciences of the United States of America, 1990, 87(87): 4576-4579. |
| 2 |
李曙光, 皮昀丹, 张传伦. 古菌研究及其展望. 中国科学技术大学学报, 2007, 37(8): 830-838. Li Shuguang, Pi Yundan, Zhang Chuanlun. The study of archaea:A review and perspectives. Journal of University of Science and Technology of China, 2007, 37(8): 830-838. |
| 3 |
曹鹏, 沈菊培, 贺纪正. 古菌细胞膜脂在古菌群落组成及其对环境响应研究中的应用. 应用生态学报, 2012, 23(9): 290-297. Cao Peng, Shen Jupei, He Jizheng. Applications of archaeal membrane lipids in investigating archaeal community composition and its responses to environmental factors. Chinese Journal of Applied Ecology, 2012, 23(9): 290-297. |
| 4 |
Koga Y, Morii H. Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Bioscience, Biotechnology, and Biochemistry, 2005, 69(11): 2019-2034. DOI:10.1271/bbb.69.2019 |
| 5 |
Matsumi R, Atomi H, Driessen A J, et al. Isoprenoid biosynthesis in Archaea——Biochemical and evolutionary implications. Research in Microbiology, 2011, 162(1): 39-52. DOI:10.1016/j.resmic.2010.10.003 |
| 6 |
Gliozzi A, Paoli G, Rosa M D, et al. Effect of isoprenoid cyclization on the transition temperature of lipid in thermophilic archaebacteria. Biochimica et Biophysica Acta(BBA)-Biomembranes, 1983, 735(2): 234-242. DOI:10.1016/0005-2736(83)90298-5 |
| 7 |
De Rosa M, Gambacorta A. The lipids of archaeabacteria. Progress in Lipid Research, 1988, 27(3): 153-175. DOI:10.1016/0163-7827(88)90011-2 |
| 8 |
Uda I, Sugai A, Itoh Y H, et al. Variation in molecular species of polar lipids from Thermoplasma acidophilum, depends on growth temperature. Lipids, 2001, 36(1): 103-105. DOI:10.1007/s11745-001-0914-2 |
| 9 |
Schouten S, Mt V D M, Hopmans E C, et al. Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in hot springs of Yellowstone National Park. Applied and Environmental Microbiology, 2007, 73(19): 6181-6191. DOI:10.1128/AEM.00630-07 |
| 10 |
Zhang Y G, Zhang C L, Liu X L, et al. Methane Index:A tetraether archaeal lipid biomarker indicator for detecting the instability of marine gas hydrates. Earth and Planetary Science Letters, 2011, 307(3): 525-534. |
| 11 |
Sinninghe Damsté J S, Rijpstra W I C, Hopmans E C, et al. Intact polar and core glycerol dibiphytanyl glycerol tetraether lipids of Group Ⅰ.1a and Ⅰ.1b Thaumarchaeota in soil. Applied and Environmental Microbiology, 2012, 78(19): 6866-6874. DOI:10.1128/AEM.01681-12 |
| 12 |
Xie W, Zhang C, Ma C. Temporal variation in community structure and lipid composition of Thaumarchaeota from subtropical soil:Insight into proposing a new soil pH proxy. Organic Geochemistry, 2015, 83-84: 54-64. DOI:10.1016/j.orggeochem.2015.02.009 |
| 13 |
Schouten S, Hopmans E C, Sinninghe Damsté J S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids:A review. Organic Geochemistry, 2013, 54: 19-61. DOI:10.1016/j.orggeochem.2012.09.006 |
| 14 |
Kuypers M M, Blokker P, Erbacher J, et al. Massive expansion of marine archaea during a Mid-Cretaceous oceanic anoxic event. Science, 2001, 293(5527): 92-95. DOI:10.1126/science.1058424 |
| 15 |
Carrillo-Hernandez T, Schaeffer P, Adam P et al. Remarkably well preserved archaeal and bacterial membrane lipids in 140 million years old sediment from the Russian platform(Kasphpir oil shales, upper Jurassic). In:21st International Meeting on Organic Geochemistry(IMOG 2003) Krakow, Books of Abstract, Part Ⅰ, 2003. 77~78
|
| 16 |
Schouten S, Hopmans E C, Forster A, et al. Extremely high sea-surface temperatures at low latitudes during the Middle Cretaceous as revealed by archaeal membrane lipids. Geology, 2003, 31(12): 1069-1072. DOI:10.1130/G19876.1 |
| 17 |
Jenkyns H C, Schouten-Huibers L, Schouten S, et al. Warm Middle Jurassic-Early Cretaceous high-latitude sea-surface temperatures from the Southern Ocean. Climate of the Past, 2012, 8(1): 215-226. DOI:10.5194/cp-8-215-2012 |
| 18 |
Schouten S, Hopmans E C, Schefuß E, et al. Distributional variations in marine crenarchaeotal membrane lipids:A new tool for reconstructing ancient sea water temperatures?. Earth and Planetary Science Letters, 2002, 204(1-2): 265-274. DOI:10.1016/S0012-821X(02)00979-2 |
| 19 |
Hopmans E C, Weijers J W H, Schefuss E, et al. A novel proxy for terrestrial organic matter in sediments based on branched and isoprenoid tetraether lipids. Earth and Planetary Science Letters, 2004, 224(1-2): 107-116. DOI:10.1016/j.epsl.2004.05.012 |
| 20 |
Turich C, Freeman K H. Archaeal lipids record paleosalinity in hypersaline systems. Organic Geochemistry, 2011, 42(9): 1147-1157. |
| 21 |
Xie S, Pancost R D, Chen L, et al. Microbial lipid records of highly alkaline deposits and enhanced aridity associated with significant uplift of the Tibetan Plateau in the Late Miocene. Geology, 2012, 40(4): 291-294. DOI:10.1130/G32570.1 |
| 22 |
丁伟华, 杨欢, 何钢强, 等. 实验模拟氧化条件对微生物四醚脂的环境替代指标的影响. 第四纪研究, 2013, 33(1): 39-47. Ding Weihua, Yang Huan, He Gangqiang, et al. Effects of oxidative degradation by hydrogen peroxide on tetraethers-based organic proxies. Quaternary Sciences, 2013, 33(1): 39-47. |
| 23 |
李奇缘, 刘潇敏, 王章章, 等. 青藏高原东部现代泥炭GDGTs分布特征及环境意义. 第四纪研究, 2016, 36(2): 388-395. Li Qiyuan, Liu Xiaomin, Wang Zhangzhang, et al. Distributions and environmental significance of GDGTs in modern peat samples from eastern Tibetan Plateau. Quaternary Sciences, 2016, 36(2): 388-395. |
| 24 |
Liu X L, Summons R E, Hinrichs K U. Extending the known range of glycerol ether lipids in the environment:Structural assignments based on tandem mass spectral fragmentation patterns. Rapid Communications in Mass Spectrometry, 2012, 26(19): 2295-2302. DOI:10.1002/rcm.6355 |
| 25 |
Liu X L, Lipp J S, Simpson J H, et al. Mono-and dihydroxyl glycerol dibiphytanyl glycerol tetraethers in marine sediments:Identification of both core and intact polar lipid forms. Geochimica et Cosmochimica Acta, 2012, 89(4): 102-115. |
| 26 |
Huguet C, Fietz S, Rosell-Melé A. Global distribution patterns of hydroxy glycerol dialkyl glycerol tetraethers. Organic Geochemistry, 2013, 57(4): 107-118. |
| 27 |
Fietz S, Huguet C, Rueda G, et al. Hydroxylated isoprenoidal GDGTs in the Nordic Seas. Marine Chemistry, 2013, 152(2): 1-10. |
| 28 |
Kaiser J, Arz H W. Sources of sedimentary biomarkers and proxies with potential paleoenvironmental significance for the Baltic Sea. Continental Shelf Research, 2016, 122: 102-119. DOI:10.1016/j.csr.2016.03.020 |
| 29 |
Lü X, Liu X L, Elling F J, et al. Hydroxylated isoprenoid GDGTs in Chinese coastal seas and their potential as a paleotemperature proxy for mid-to-low latitude marginal seas. Organic Geochemistry, 2015, 89-90: 31-43. DOI:10.1016/j.orggeochem.2015.10.004 |
| 30 |
Fietz S, Ho S L, Huguet C, et al. Appraising GDGT-based seawater temperature indices in the Southern Ocean. Organic Geochemistry, 2016, 102: 93-105. DOI:10.1016/j.orggeochem.2016.10.003 |
| 31 |
Knies J, Cabedosanz P, Belt S T, et al. The emergence of modern sea ice cover in the Arctic Ocean. Nature Communications, 2014, 5(5608): 5608-5608. |
| 32 |
Xie S, Liu X L, Schubotz F, et al. Distribution of glycerol ether lipids in the oxygen minimum zone of the eastern tropical North Pacific Ocean. Organic Geochemistry, 2014, 71(6): 60-71. |
| 33 |
Zhu C, Wakeham S G, Elling F J, et al. Stratification of archaeal membrane lipids in the ocean and implications for adaptation and chemotaxonomy of planktonic archaea. Environmental Microbiology, 2016, 18(12): 4324-4336. DOI:10.1111/1462-2920.13289 |
| 34 |
An Z, Porter S C, Kutzbach J E, et al. Asynchronous Holocene Optimum of the East Asian monsoon. Quaternary Science Reviews, 2000, 19(8): 743-762. DOI:10.1016/S0277-3791(99)00031-1 |
| 35 |
Shen J, Liu X, Wang S, et al. Palaeoclimatic changes in the Qinghai Lake area during the last 18, 000 years. Quaternary International, 2005, 136(1): 131-140. DOI:10.1016/j.quaint.2004.11.014 |
| 36 |
侯居峙, WilliamJ D'Andrea, 柳中晖. 湖泊碳库效应对青藏高原气候变化解释的影响探讨. 第四纪研究, 2012, 32(3): 441-453. Hou Juzhi, William J D'Andrea, Liu Zhonghui. Geochronological limitations for interpreting the paleoclimatic history of the Tibetan Plateau. Quaternary Sciences, 2012, 32(3): 441-453. |
| 37 |
张彭熹, 张保珍, 钱桂敏, 等. 青海湖全新世以来古环境参数的研究. 第四纪研究, 1994(3): 225-238. Zhang Pengxi, Zhang Baozhen, Qian Guimin, et al. The study of paleoclimatic parameter of Qinghai Lake since Holocene. Quaternary Sciences, 1994(3): 225-238. |
| 38 |
Zhang J, Jin M, Chen F, et al. High-resolution precipitation variations in the northeast Tibetan Plateau over the last 800 years documented by sediment cores of Qinghai Lake. Chinese Science Bulletin, 2003, 48(14): 1451-1456. DOI:10.1360/02wd0271 |
| 39 |
Zhang E, Ji S, Wang S, et al. Quantitative reconstruction of the paleosalinity at Qinghai Lake in the past 900 years. Chinese Science Bulletin, 2004, 49(7): 730-734. DOI:10.1007/BF03184273 |
| 40 |
Liu Z, Henderson A C G, Huang Y. Alkenone-based reconstruction of Late-Holocene surface temperature and salinity changes in Lake Qinghai, China. Geophysical Research Letters, 2006, 33(9): 370-386. |
| 41 |
Liu X Q, Shen J, Wang S M, et al. Southwest monsoon changes indicated by oxygen isotope of ostracode shells from sediments in Qinghai Lake since the Late Glacial. Chinese Science Bulletin, 2007, 52(4): 539-544. DOI:10.1007/s11434-007-0086-3 |
| 42 |
Henderson A C G, Holmes J A. Palaeolimnological evidence for environmental change over the past millennium from Lake Qinghai sediments:A review and future research prospective. Quaternary International, 2009, 194(1-2): 134-147. DOI:10.1016/j.quaint.2008.09.008 |
| 43 |
An Z, Colman S M, Zhou W, et al. Interplay between the Westerlies and Asian monsoon recorded in Lake Qinghai sediments since 32ka. Scientific Reports, 2012, 2(8): 619. |
| 44 |
Jin Z, An Z, Yu J, et al. Lake Qinghai sediment geochemistry linked to hydroclimate variability since the Last Glacial. Quaternary Science Reviews, 2015, 122: 63-73. DOI:10.1016/j.quascirev.2015.05.015 |
| 45 |
Chen F, Wu D, Chen J, et al. Holocene moisture and East Asian summer monsoon evolution in the northeastern Tibetan Plateau recorded by Lake Qinghai and its environs:A review of conflicting proxies. Quaternary Science Reviews, 2016, 154: 111-129. DOI:10.1016/j.quascirev.2016.10.021 |
| 46 |
Li G, Dong H, Hou W, et al. Temporal succession of ancient phytoplankton community in Qinghai Lake and implication for paleo-environmental change. Scientific Reports, 2016, 6: 19769. DOI:10.1038/srep19769 |
| 47 |
Zhou W, Liu T, Wang H, et al. Geological record of meltwater events at Qinghai Lake, China from the past 40ka. Quaternary Science Reviews, 2016, 149: 279-287. DOI:10.1016/j.quascirev.2016.08.005 |
| 48 |
Liu W, Liu Z, Wang H, et al. Salinity control on long-chain alkenone distributions in lake surface waters and sediments of the northern Qinghai-Tibetan Plateau, China. Geochimica et Cosmochimica Acta, 2011, 75(7): 1693-1703. DOI:10.1016/j.gca.2010.10.029 |
| 49 |
Jin Z, You C F, Wang Y, et al. Hydrological and solute budgets of Lake Qinghai, the largest lake on the Tibetan Plateau. Quaternary International, 2010, 218(1): 151-156. |
| 50 |
Lister G S, Kelts K, Zao C K, et al. Lake Qinghai, China:Closed-basin like levels and the oxygen isotope record for ostracoda since the Latest Pleistocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 1991, 84(1): 141-162. |
| 51 |
Williams W D. Chinese and Mongolian saline lakes:A limnological overview. Hydrobiologia, 1991, 210(1): 39-66. |
| 52 |
Colman S M, Yu S Y, An Z, et al. Late Cenozoic climate changes in China's western interior:A review of research on Lake Qinghai and comparison with other records. Quaternary Science Reviews, 2007, 26(17-18): 2281-2300. DOI:10.1016/j.quascirev.2007.05.002 |
| 53 |
Li X Y, Xu H Y, Sun Y L, et al. Lake-level change and water balance analysis at Lake Qinghai, West China during recent decades. Water Resources Management, 2007, 21(9): 1505-1516. DOI:10.1007/s11269-006-9096-1 |
| 54 |
Xiao J, Jin Z D, Zhang F, et al. Solute geochemistry and its sources of the groundwaters in the Qinghai Lake catchment, NW China. Journal of Asian Earth Sciences, 2012, 52(4): 21-30. |
| 55 |
Wang H, Dong H, Zhang C L, et al. Water depth affecting thaumarchaeol production in Lake Qinghai, northeastern Qinghai-Tibetan Plateau:Implications for paleo lake levels and paleoclimate. Chemical Geology, 2014, 368(4): 76-84. |
| 56 |
Wang H, Dong H, Zhang C L, et al. Deglacial and Holocene archaeal lipid-inferred paleohydrology and paleotemperature history of Lake Qinghai, northeastern Qinghai-Tibetan Plateau. Quaternary Research, 2015, 83(1): 116-126. DOI:10.1016/j.yqres.2014.10.003 |
| 57 |
Wang H Y, Dong H L, Zhang C L, et al. A 12-kyr record of microbial branched and isoprenoid tetraether index in Lake Qinghai, northeastern Qinghai-Tibet Plateau:Implications for paleoclimate reconstruction. Science China:Earth Sciences, 2016, 59(5): 951-960. DOI:10.1007/s11430-015-5213-4 |
| 58 |
Schouten S, Hopmans E C, Baas M, et al. Intact membrane lipids of "Candidatus Nitrosopumilus maritimus, " a cultivated representative of the cosmopolitan mesophilic group Ⅰ Crenarchaeota. Applied and Environmental Microbiology, 2008, 74(8): 2433-2440. DOI:10.1128/AEM.01709-07 |
| 59 |
Pitcher A, Hopmans E C, Mosier A C, et al. Core and intact polar glycerol dibiphytanyl glycerol tetraether lipids of ammonia-oxidizing archaea enriched from marine and estuarine sediments. Applied and Environmental Microbiology, 2011, 77(10): 3468-3477. DOI:10.1128/AEM.02758-10 |
| 60 |
Elling F J, Könneke M, Lipp J S, et al. Effects of growth phase on the membrane lipid composition of the thaumarchaeon Nitrosopumilus maritimus, and their implications for archaeal lipid distributions in the marine environment. Geochimica et Cosmochimica Acta, 2014, 141: 579-597. DOI:10.1016/j.gca.2014.07.005 |
| 61 |
Elling F J, Könneke M, Mußmann M, et al. Influence of temperature, pH, and salinity on membrane lipid composition and TEX86 of marine planktonic thaumarchaeal isolates. Geochimica et Cosmochimica Acta, 2015, 171: 238-255. DOI:10.1016/j.gca.2015.09.004 |
| 62 |
Sinninghe Damsté J S, Schouten S, Hopmans E C, et al. Crenarchaeol:The characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota. Journal of Lipid Research, 2002, 43(10): 1641-1651. DOI:10.1194/jlr.M200148-JLR200 |
| 63 |
Brochier-Armanet C, Boussau B, Gribaldo S, et al. Mesophilic crenarchaeota:Proposal for a third archaeal phylum, the Thaumarchaeota. Nature Reviews Microbiology, 2008, 6(3): 245-252. DOI:10.1038/nrmicro1852 |
| 64 |
Pitcher A, Rychlik N, Hopmans E C, et al. Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic Group Ⅰ.1b Archaeon. ISME Journal, 2010, 4(4): 542-552. DOI:10.1038/ismej.2009.138 |
| 65 |
Koga Y, Akagawa-Matsushita M, Ohga M, et al. Taxonomic significance of the distribution of component parts of polar ether lipids in Methanogens. Systematic and Applied Microbiology, 1993, 16(3): 342-351. DOI:10.1016/S0723-2020(11)80264-X |
| 66 |
Gambacorta A, Gliozzi A, Rosa M D. Archaeal lipids and their biotechnological applications. World Journal of Microbiology and Biotechnology, 1995, 11(1): 115-131. DOI:10.1007/BF00339140 |
| 67 |
Yang J, Jiang H, Dong H, et al. Sedimentary archaeal amoA gene abundance reflects historic nutrient level and salinity fluctuations in Qinghai Lake, Tibetan Plateau. Scientific Reports, 2015, 5: 18071. |
| 68 |
Auguet J C, Barberan A, Casamayor E O. Global ecological patterns in uncultured Archaea. ISME Journal, 2010, 4(2): 182-190. DOI:10.1038/ismej.2009.109 |
| 69 |
Bates S T, Berg-Lyons D, Caporaso J G, et al. Examining the global distribution of dominant archaeal populations in soil. ISME Journal, 2011, 5(5): 908-917. DOI:10.1038/ismej.2010.171 |
| 70 |
Huguet C, Fietz S, Rosell-Melé A, et al. Molecular dynamics simulation study of the effect of glycerol dialkyl glycerol tetraether hydroxylation on membrane thermostability. Biochimica et Biophysica Acta(BBA)-Biomembranes, 2017, 1859(5): 966-974. DOI:10.1016/j.bbamem.2017.02.009 |
| 71 |
李凡, 侯光良, 鄂崇毅, 等. 青藏高原全新世气温序列的集成重建. 干旱区研究, 2015, 32(4): 716-725. Li Fan, Hou Guangliang, E Chongyi, et al. intergrated reconstruction of the Holocene temperature series of Qinghai-Tibet Plateau. Arid Zone Research, 2015, 32(4): 716-725. |
| 72 |
Pouliot J, Galand P E, Lovejoy C, et al. Vertical structure of archaeal communities and the distribution of ammonia monooxygenase A gene variants in two meromictic High Arctic lakes. Environmental Microbiology, 2009, 11(3): 687-699. DOI:10.1111/emi.2009.11.issue-3 |
| 73 |
Buckles L K, Villanueva L, Weijers J W H, et al. Linking isoprenoidal GDGT membrane lipid distributions with gene abundances of ammonia-oxidizing Thaumarchaeota and uncultured crenarchaeotal groups in the water column of a tropical lake(Lake Challa, East Africa). Environmental Microbiology, 2013, 15(9): 2445-2462. DOI:10.1111/emi.2013.15.issue-9 |
| 74 |
Tierney J E, Russell J M, Eggermont H, et al. Environmental controls on branched tetraether lipid distributions in tropical East African lake sediments. Geochimica et Cosmochimica Acta, 2010, 74(17): 4902-4918. DOI:10.1016/j.gca.2010.06.002 |
| 75 |
Merbt S N, Stahl D A, Casamayor E O, et al. Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiology Letters, 2012, 327(1): 41. DOI:10.1111/fml.2012.327.issue-1 |
| 76 |
Liu X, Lai Z, Madsen D, et al. Last deglacial and Holocene lake level variations of Qinghai Lake, northeastern Qinghai-Tibetan Plateau. Journal of Quaternary Sciences, 2015, 30(3): 245-257. DOI:10.1002/jqs.v30.3 |
| 77 |
Liu W, Li X, An Z, et al. Total organic carbon isotopes:A novel proxy of lake level from Lake Qinghai in the Qinghai-Tibet Plateau, China. Chemical Geology, 2013, 347: 153-160. DOI:10.1016/j.chemgeo.2013.04.009 |
| 78 |
刘卫国, 王政, 李祥忠. 内源贡献对青海湖碳同位素指标量化的影响. 第四纪研究, 2016, 36(3): 623-629. Liu Weiguo, Wang Zheng, Li Xiangzhong. The contribution of aquatic plants to sedimentary n-alkanes δ13C values using to qualify compositions of terrigenous plants in Lake Qinghai on the northeastern Qinghai-Tibetan Plateau. Quaternary Sciences, 2016, 36(3): 623-629. |
| 79 |
Karner M B, Delong E F, Karl D M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature, 2001, 409(6819): 507-510. DOI:10.1038/35054051 |
| 80 |
Jiang H, Dong H, Yu B, et al. Dominance of putative marine benthic Archaea, in Qinghai Lake, north-western China. Environmental Microbiology, 2008, 10(9): 2355-2367. DOI:10.1111/emi.2008.10.issue-9 |
| 81 |
Jiang H C, Dong H L, Deng S C, et al. Response of archaeal community structure to environmental changes in lakes on the Tibetan Plateau, Northwestern China. Geomicrobiology Journal, 2009, 26(4): 289-297. DOI:10.1080/01490450902892662 |
| 82 |
Ye W, Liu X, Lin S, et al. The vertical distribution of bacterial and archaeal communities in the water and sediment of Lake Taihu. FEMS Microbiology Ecology, 2009, 70(2): 107-120. |
② Department of Marine Science and Engineering, Southern University of Science and Technology, Shenzhen 518055;
③ State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences(Beijing), Beijing 100083;
④ State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences(Wuhan), Wuhan 430074)
Abstract
Archaeal lipids are ubiquitous in natural environments and have been proven to be useful biomarkers for paleoclimate studies. Recently, a new group of archaeal lipids, i.e., hydroxylated glycerol dialkyl glycerol tetraethers(OH-GDGTs)with one or two hydroxyl groups in the biphytanyl moieties of isoprenoid GDGTs(isoGDGTs), was identified from marine sediments. Subsequent studies found increasing OH-GDGT contributions towards higher latitudes and lower water temperatures in marine and lake sediments, and therefore, the relative abundances of OH-GDGTs are potential proxies for paleotemperature reconstruction. Until now, however, knowledge is still limited about their occurrence and distribution in lakes. Lake Qinghai, located at the transition from the arid to the semi-arid climate zones, is the largest interior plateau lake in Central Asia. Due to the sensitivity of its regional climate to monsoon variation and global change, paleoclimate reconstructions from Lake Qinghai are of wide interest for understanding how complex forcing mechanisms could affect the regional climate. Hence, the development and validation of novel proxies for paleoclimate reconstructions are of great importance for this lake. In this study, we investigated the occurrence and distribution of OH-GDGTs in 12 downcore sediment samples(collection at 0~337cm in depth)of core QH-2011(36°39'34"N, 100°35'37"E) with a whole length of ca. 5.8 m. This core was recovered at a water depth of ca.24 m in the southeastern sub-basin of Lake Qinghai and the samples used in this study covered the Middle and Late Holocene periods(past ca. 8ka). The total lipid was extracted ultrasonically by using MeOH:Dichloromethane(9:1, v/v)for 3 times, and fractionated over an activated silica gel column. The polar fraction containing OH-GDGTs was measured using high-performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. The results showed that OH-GDGTs were generally abundant in the sediments of Lake Qinghai during the past 8ka, with OH-GDGT-0 being the most abundant. The concentration of OH-GDGTs ranged from 0.8ng/g to 53.6ng/g, while the relative abundance of OH-GDGTs(to the sum of isoGDGTs and OH-GDGTs)ranged from 0.56% to 10.28%, both of which were relatively low in the early Middle Holocene sediments and high in the Late Holocene sediments. The fractional abundance of OH-GDGTs was positively correlated with the fractional abundance of crenarchaeol, a unique archaeal GDGT specifically from Thaumarchaeota(R2=0.93; p < 0.01), while the variation in the concentration of OH-GDGTs resembled that for thaumarchaeotal amoA gene abundance, pointing to a Thaumarchaeotal origin of OH-GDGTs in Lake Qinghai. Since the producer of OH-GDGTs, i.e., Thaumarchaeota prefers living in stratified waters with a deep mixed layer, where ammonium availability near the oxycline is high and the competition with photoautotrophs is low, we speculate that variations in OH-GDGT concentration and% OH-GDGTs are possibly controlled by changes in lake water depth. Further comparison between OH-GDGTs(both concentration and relative abundance)and lake level proxies(including shoreline evidences, the relative abundance of crenarchaeol, and total organic carbon isotopes)supports that lake water depth plays an important role in affecting archaeal community structure and the production of OH-GDGTs. Therefore, we propose that OH-GDGTs preserved in lacustrine sediments might help to reconstruct the paleohydrological history of inland lakes. 2017, Vol.37
2017, Vol.37
