文章信息
- 高亚辉, 尹国杰, 张少文, 王璐, 孟巧静, 李欣栋
- GAO Ya-hui, YIN Guo-jie, ZHANG Shao-wen, WANG Lu, MENG Qiao-jing, LI Xin-dong
- 电化学法制备石墨烯的研究进展
- Research progress in electrochemical preparation of graphene
- 材料工程, 2020, 48(8): 84-100
- Journal of Materials Engineering, 2020, 48(8): 84-100.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2019.000704
-
文章历史
- 收稿日期: 2019-07-26
- 修订日期: 2019-10-09
2. 洛阳理工学院 河南省重金属污染土壤修复工程技术研究中心, 河南 洛阳 471023
2. Henan Engineering Technology Research Center of Remediation of Heavy Metal Contaminated Soil, Luoyang Institute of Science and Technology, Luoyang 471023, Henan, China
石墨烯是一种由碳原子以sp2杂化构成的六边形点阵结构2D层状纳米材料,具有电子迁移率高、比表面积大、透光率高和化学性能稳定等特性,在储能转化、电子器件、工业催化、传感器和生物医疗等领域具有巨大的应用前景[1]。自英国曼彻斯特大学的Novoselov等[2]首次采用微机械法从石墨中剥离出单层石墨烯以来,目前已开发形成了微机械剥离法、化学气相沉积(CVD)法、化学氧化还原法、液相剥离法等十多种制备技术,但均不够成熟。微机械剥离法因石墨烯产率低和控制性差限制了其工业化应用[3-4];化学氧化还原法虽可实现石墨烯规模化生产,但因所用强氧化剂或活性化合物不可逆地破坏了石墨烯晶体结构而使其应用性能明显降低,同时在生产过程中还存在爆炸危险和严重的污染问题[5-9];CVD法虽然是生产大面积石墨烯薄膜的技术,但由于工艺复杂、成本高等问题限制了其大规模生产和应用[10-14];常规液相剥离法虽然具有成本低、操作流程简单、产物结构完整等优势,但石墨烯剥离产率比较低[15-21]。因此,开发高效率、规模化、低成本和绿色环保的石墨烯制备技术是其应用发展的关键。
电化学剥离是近年来开发的一种石墨烯制备新方法,其主要是通过电解驱动异性带电离子及混合物插入石墨电极,膨胀剥落石墨间层后获得石墨烯[22-23],该方法主要优势是:石墨原料储量丰富、成本低廉;电压及电流大小能够精确调节,石墨剥离过程具有可重现性和可操作性;电解通常在室温或接近室温的温和条件下进行,易于控制[24]。基于此,本文对目前最具发展潜力的电化学法制备石墨烯(EG)技术的研究进行了综述,阐述了电化学法制备石墨烯的机制,重点分析了石墨阳极氧化和阴极剥离过程涉及因素变化对石墨烯的剥离效率及产率、形貌、质量和缺陷程度的影响,同时简要介绍了电化学法制备功能化石墨烯材料及其应用,最后展望了电化学法制备石墨烯技术的未来发展方向。
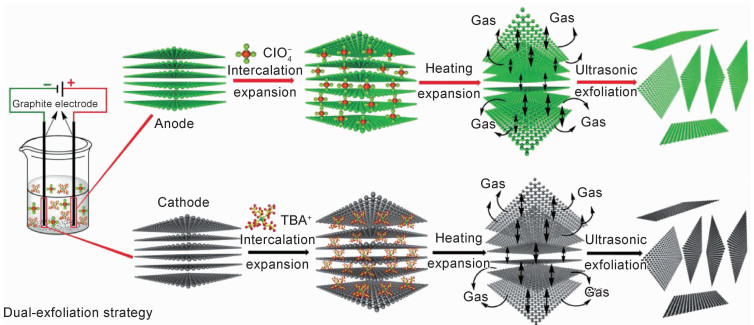
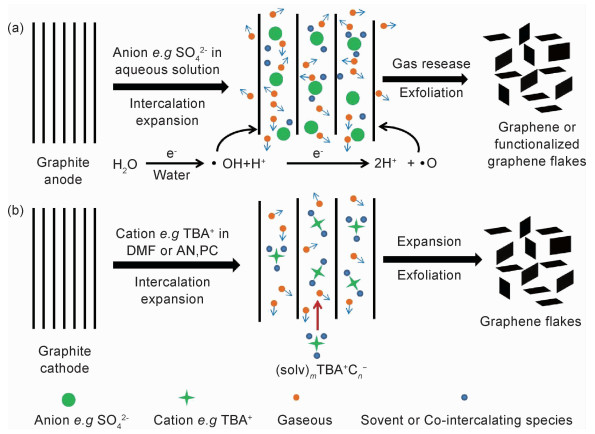
1 电化学法制备石墨烯的机制电化学制备石墨烯分阳极氧化剥离和阴极插层剥离两种方法,均是将石墨作为阳极(或阴极),通过施加外来电压驱动电解质中阴离子(或溶剂化的阳离子)有效嵌入石墨阳极(或阴极)层间,形成石墨插层复合物(GIC),并通过GIC氧化或还原反应产生气体的膨胀力优先打开石墨层间范德华力较弱的晶界、边缘和其他缺陷位置,使石墨层微观结构大比例膨胀和有效剥落,最终实现石墨烯的制备[25-28],其制备过程如图 1所示。
|
图 1 电化学插层剥离石墨阳极(a)和阴极(b)制备石墨烯的机理示意图 Fig. 1 Schematic illustration of graphite anode (a) and cathode (b) electrochemical intercalation exfoliation mechanisms for graphene |
石墨阳极氧化过程中,阳极处阴离子分解产生和逸出的气体使石墨膨胀和剥落的速率较快,但是,当阳极电位较高(如+10 V)时,电解液中的水会失去电子产生羟基(·OH)和氧(·O)自由基(图 1(a)),这些活泼的自由基将氧化石墨的晶界、边缘位置,同时产生的气体逸出也会使石墨膨胀和剥落,因此阳极氧化剥离石墨制备的石墨烯通常包含有羟基、羰基和环氧基等含氧基团,使其结构存在一定的缺陷。与此相反,石墨阴极插层剥离通常是在有机溶剂或离子液体等无水体系中进行,虽然石墨膨胀和剥离的速率较慢,但可避免水电解产生强氧化性物质氧化电极而造成石墨结构破坏,因此,可制备高质量的石墨烯(图 1(b))。采用电化学法剥离石墨制备石墨烯过程中,离子类型及浓度、溶剂、电压或电流和温度等参数对石墨烯的剥离效率和产率、形貌、质量和缺陷密度均会产生影响[29]。
2 石墨阳极氧化 2.1 剥离效率和产率电化学法制备石墨烯的剥离效率、产率与电解质溶液中阴离子的种类和大小有关。2011年,Su等[30]以H2SO4和KOH混合液作为电解质剥离石墨阳极时,产率仅为5%~8%(质量分数,下同)。之后,Parvez等以H2SO4溶液作为电解质时,阳极石墨棒脱落黑色粉末形成溶液只需10 min,其产率约为60%[31]。与此对比,以H3PO4和H2C2O4质子酸作为电解质时,剥离速率和效率较低;以硫酸盐Na2SO4,(NH4)2SO4和K2SO4作为电解质时,电解10 min其产率均可达到75%[32]。以LiClO4盐作为电解质时,电解1 h后仅观察到石墨箔处于膨胀状态而没有进一步剥离;其他如以硝酸和硝酸盐[33-34]溶液作为电解质时,NO3-和H2O分子插层进入石墨层间,平面结构的NO3-会自发形成NO2+进而氧化石墨边缘,虽可获得产率达76%的功能化石墨烯,但剥离时间通常需几十个小时,剥离效率较低。综合比对,采用硫酸和含硫酸根的盐作为电解质比采用其他盐类作为电解质时,石墨烯的剥离效率和产率更高[35-37]。究其原因,主要是SO42-大小(0.46 nm)与石墨层间距(0.335 nm)相近,较其他阴离子更易插入石墨层空间,且水电解产生自由基(·OH和·O)的氧化作用及SO42-分解逸出的SO2气体在石墨膨胀和剥离中起重要作用。
阳极氧化制备石墨烯时,在电解质溶液中添加辅助试剂有助于提高石墨烯的剥离产率。如将三聚氰胺分子添加在H2SO4电解质中,可通过—NH2吸附在石墨表面形成的亲水作用力诱导SO42-嵌入石墨,进而可获得产率达到80%的3层以下石墨烯[38];将H2O2添加在NaOH碱性电解质中,H2O2分子会与OH-反应生成具有强亲核性的过氧离子(O22-),进而可获得产率高达95%的高质量石墨烯[39]。
电化学法制备石墨烯时,加热电解质将对石墨烯的剥离效率、产率产生两方面影响:一是温度升高会使石墨层间距扩大,便于阴离子插入层间;二是加热电解质会对离子热振动产生影响。Tripathi等[40]采用H2SO4/KOH/去离子水作为电解液,当温度从室温升至80 ℃时,石墨烯产率从17%显著增大到77%,总体约提高了4.5倍。这是因为当电解液温度升高到80 ℃并处于稳定状态时,石墨在底部边缘或晶界位置产生热膨胀,层间距从室温时的0.335 nm增大到80 ℃时的0.336 nm,促使阴离子SO42-快速嵌入和石墨层连续打开,进而层与层之间的范德华作用力减弱,最终剥离成石墨烯。同时,随着电解液温度升高,各向同性热振动会增大,当不断增强的离子平面热振动受到抑制时,垂直于石墨底部的离子热振动会不断增强,对石墨边缘产生垂直连续冲击作用力,从而使石墨烯的剥离效率和产率增加。此外,石墨层间以弱范德华力结合,其结合能约为(31±2) meV[41],而电解液被加热到最高温度80 ℃时的热能约30 meV,二者十分接近,这说明加热电解质引起的离子热能增加可能会削弱或破坏石墨层间的范德华力。因此,阳极石墨电化学剥离的产率受电解液温度的影响显著。
电化学法制备石墨烯的剥离效率还受电解电压或电流密度的影响,一般较高的电解电压或电流密度会增大石墨烯的剥离效率。Coros等[42]报道了在H2SO4和HNO3组成的电解质中,当电压从2.5 V升高到6 V时,阳极石墨剥离成石墨烯的产率从零增加到约55%。Kumar等[43]以糖精钠溶液为电解质,当电解电压从2 V逐步增加到10 V时,石墨烯产率也随着电位升高而不断增大。
2.2 石墨烯形貌阳极石墨电化学剥离时通常分为阴离子插层、石墨膨胀和剥离三个阶段。在较高外加电压或电流密度下,水电解产生氧化性自由基和大量气体会加剧石墨膨胀和分层,导致其在剥离之前出现无效电流供给或断路,造成剥落的石墨烯较厚(多于5层)。为得到较薄的石墨烯,一般需采用超声波辅助进一步剥离,但同时会使石墨烯尺寸减小(小于5 μm)[44]。因此,为获得较好的石墨烯形貌,选择合适的电解液体系和剥离条件十分重要。钟轶良[45]选择(NH4)2HPO4电解液,通过带正电石墨吸附PO43-,诱导使其插入石墨层间并促使结构膨胀,最终获得了尺寸达20 μm、厚度约3 nm的石墨烯。
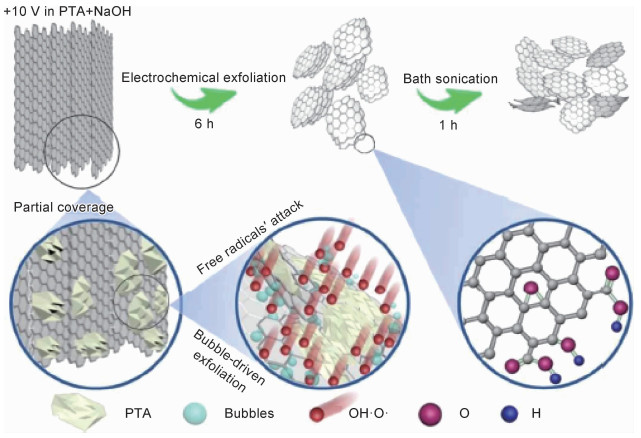
中国科学院上海微系统研究所丁古巧团队[46-47]选用高定向热解石墨(HOPG)代替石墨棒作为阳极,以NaOH与邻苯二甲酸(PTA)混合液作为电解体系,在无需超声处理的情况下,实现了尺寸范围为1~50 μm、层数为1~3层(78.94%)石墨烯的制备,这是传统电化学法制备效率的10倍。机理研究表明:在外加电压下,阳极周围pH值的局部降低(4OH- →2H2O+4e-+O2)会使附近的PTA分子从NaOH中沉积出来并覆盖在部分石墨表面,从而使有效剥离面积和电流密度减小,进一步导致水电解产生氧气泡和石墨剥离过程变得缓慢。同时,在阳极处产生的HO·和O·会选择性地剥蚀没有PTA覆盖的石墨边缘,析出的O2会在腐蚀的石墨边缘产生剪切力,促使石墨剥落和逐层剥离(如图 2所示)。作者认为电解质(NaOH和PTA)的合理设计和阳极HOPG的选择,实现了阳极氧化增强和石墨前体的大尺寸剥离。因此,在阳极氧化电化学制备石墨烯中,电解质体系和石墨阳极的合理选择对石墨烯形貌起着非常重要的作用。
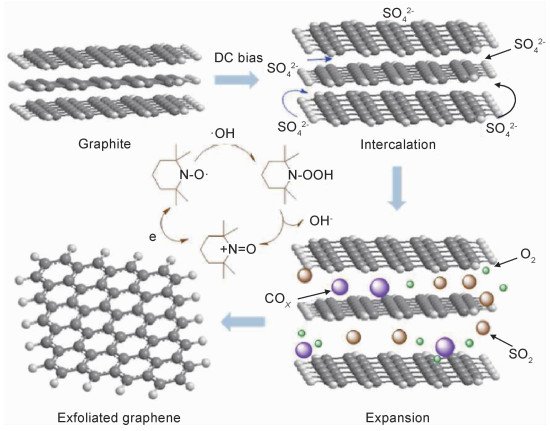
EG按照含氧量不同分为高质量石墨烯、水溶性石墨烯和氧化石墨烯(EGO)三类。在质子酸或无机盐水溶液电解质中,阳极电位低于+10 V,室温剥离2~30 min,所得石墨烯含氧量的原子分数约为10%[30-35],其中,水电解产生OH·和O·自由基的氧化作用是石墨烯缺陷形成的根本原因。为了提高石墨烯的质量,研究者选择一系列的还原剂作为添加剂,例如2, 2, 6, 6-四甲基哌啶-氮-氧化物(TEMPO)、抗坏血酸和硼氢化钠等,以消除这些自由基和控制石墨剥离过程。Yang等[48]在(NH4)2SO4电解质溶液中添加TEMPO,水电解产生的HO·会立即与周边的TEMPO反应,形成亚稳态的中间体(TEMPO-OH),并转化为氧甲铵阳离子,进而抑制石墨过度氧化,同时电化学剧烈反应析出的多种气体(如COX,SO2和O2)显著削弱了石墨层间的范德华力,使最终制备的高质量石墨烯平均尺寸为5~10 μm,ID/IG值低于0.1,碳氧比(C/O)约为25.3,且具有优异电子迁移率(约405 cm2 ·V-1·s-1),反应机理如图 3所示。此外,三聚氰胺作为H2SO4电解质的添加剂可吸附在石墨表面,提供原位保护,防止剥离的石墨烯进一步被氧化,从而获得含氧或缺陷密度低(C/O为26.17, ID/IG < 0.45)的高质量石墨烯[38];H2O2作为(NH4)2SO4电解质的添加剂,也可使石墨烯表面的氧官能团减少[49];甘氨酸与HSO4-离子配合物作为一种有效的电解质,可在5 min内将石墨剥离成氧含量较低(C/O为89/11)的少层高质量石墨烯纳米片(GNs),远低于在稀硫酸电解质中制备石墨烯的含氧量[50]。
由于电化学法制备的石墨烯氧化程度低,通常只能分散在高沸点和有毒的有机溶剂中,这不仅限制了EG的进一步加工和功能化应用,也会引起环境污染和健康问题,因此,丁古巧团队[51]选择氧化能力较强的硫酸氢钾(HSO5·0.5KHSO4·0.5K2SO4)复合盐溶液作为电解质,阳极施加50 V电压4 min后,可将石墨箔剥离成2~5层、氧含量的原子分数约为16.37%的水溶性石墨烯。机理研究表明:硫酸氢钾复合盐溶解生成较多的SO42-,HSO5-和HSO4-阴离子,在电场作用下,SO42-作为一种理想的插层剂嵌入石墨层间,HSO5-和HSO4-阴离子会活化成具有极强氧化性的自由基SO·或OH·,氧官能团通过破坏碳原子的sp2共轭结构接枝到石墨烯表面,因此形成的石墨烯与常规化学氧化法(Hummers)制备的氧化石墨烯(HGO)一样具有相似的水分散性。此外,采用NaOH+PTA电解质体系,通过使阳极HOPG表面覆盖PTA分子来有效减小剥离面积和电流密度,并延长阳极表面氧化时间以获得氧含量的原子分数高达24.08%(C/O比为3.15)、浓度达2.5 mg·mL-1的水溶性石墨烯[46]。
电化学法制备石墨烯的质量、缺陷程度不仅与电解质有关,还与电解液温度和电流密度有关。Hsieh等[52]研究发现,产物石墨烯纳米片(GNs)晶格层间距随着温度的升高会不断增大,剥离温度对制备GNs质量也有影响;低温时,产物由单层或少层GNs组成;较高温度时,产物由表面存在许多孔洞的GNs组成,且会导致石墨烯层堆叠,不利于生产高质量的GNs[53-54]。此外,随着电流密度的增大,产物的ID/IG比值也增加[55]。因此,温度、电流密度等条件的合适选择对电化学剥离法制备石墨烯的质量有重要影响。
电解液pH值会石墨烯氧含量和缺陷密度产生影响,主要原因是电解质阴离子和中间自由基的氧化性会随电解液pH值的变化而变化。当以碱性NaOH、中性NaCl和酸性稀硫酸为电解质时,石墨烯的紫外最大吸收峰在碱性、中性和酸性电解液中分别出现在268, 260 nm和230 nm处,说明氧化程度随着pH值降低而增加。同时,在碱性、中性和酸性溶液中ID/IG比值(0.13, 0.204, 0.312)依次升高[56]。此外,将NaOH添加在含NaClO4电解液中以升高pH值后,可促使石墨烯氧官能团产生电化学还原,使其缺陷和氧含量降低,从而产生大量高质量GNs[57]。
电解质浓度会对影响石墨烯的氧化程度,并且还会影响石墨烯的剥离效率。总体来说,石墨烯的氧化程度会随着电解液浓度升高而增大。据报道,电化学剥离HOPG时,在较低浓度(0.1,0.3 mol·L-1)H2SO4电解液中,石墨不发生剥离;当H2SO4浓度增大至0.5 mol·L-1时,可获得层数为4~6层、横向尺寸为11~26 μm、形态均匀的少层石墨烯;当H2SO4浓度继续升至1.0 mol·L-1时,SO42-离子浓度增大促使石墨膨胀、析出气体量增加,进而使石墨烯横向尺寸大幅减少、表面氧化或氧含量显著增加[58]。通过研究,在不同浓度(0.5,1.0,1.5,2.0 mol·L-1)的高氯酸(HClO4)电解液剥离石墨烯也得到了类似结果,石墨烯的缺陷密度和氧含量会随HClO4浓度升高而增加[59],尤其是当HClO4浓度增加到8 mol·L-1时,即使在较低电位下,石墨阳极也会被完全氧化成石墨氧化物[60]。
在含水电解液中采用电化学法插层剥离石墨时,水分解产生气体(H2或O2)的膨胀作用会使部分未经有效插层剥离的石墨颗粒或石墨厚片从电极表面剥落下来,因其失去电接触而无法进一步氧化和剥离成石墨烯。为解决该问题,沈阳金属研究所任文才团队[61-62]提出了以柔性石墨纸作为原料的EGO两步制备法,首先在盛有浓H2SO4(98%)的电解池中插层形成GIC,再在装有稀H2SO4(50%)溶液的另一电解池中进行氧化剥离,以高效制备EGO。结果发现,经过插层的石墨纸在第二步不仅可在几秒钟内实现快速氧化,更重要的是能有效抑制电解液的水进入电极内部,减少水分解造成的无效石墨剥落,实现氧化剥离过程的连续进行。所得EGO产率约为96%,ID/IG比值高于HGO的比值,其氧化程度较高(C/O约为1.5~1.8),其氧化速率比Hummers法快100倍以上。同时,还可快速连续制备EGO并循环利用H2SO4,从而大幅减少了制备过程中的污染物排放,实现了EGO高效率、低成本和绿色环保制备。
综上所述,石墨阳极氧化制备石墨烯的效率和产率提高可通过改变电解质类型、增大电解液浓度、加入辅助添加剂来实现;也可通过升高电解液温度、增大电压或电流密度来实现,但这同时会使石墨烯的含氧量和缺陷密度升高。选择合适的电解质体系和剥离条件对控制石墨烯的形貌尺寸至关重要。通过控制电解质类型、电解液浓度、添加剂种类、电解温度、电压和电流密度等条件,除可获得高质量石墨烯、水溶性石墨烯外,还可获得EGO。研究者将制备出的EGO进一步制成了透明导电薄膜、柔性石墨烯纸和超轻气凝胶,其中导电薄膜经还原后,透明度为80%石墨烯膜的表面电阻约为1.5 kΩ/sq,与通过热还原氧化石墨烯的相似;制成的石墨烯纸机械强度为175 MPa,高于通过热还原氧化石墨烯制备的石墨烯纸[63];制备的气凝胶具有高度发达的孔结构和高弹性,它能支撑起自身1000倍以上的质量且保持结构完整,并能从超过80%的压缩中压力撤回时迅速恢复原状。基于EG组装的EG-Si电极材料,经100次循环后,可逆容量仍然高达1310 mAh/g(容量保持率为86%),而还原氧化石墨烯(RGO)-Si的可逆容量仅为748 mAh/g(容量保持率为53%)[64]。采用表面包覆聚多巴胺的EG薄膜组装的微型超级电容器展现出体积电容达340 F/cm3,功率密度达1000 W/cm3的优异电化学性能,优于已报道的大多数石墨烯基微超级电容器[65]。此外,通过喷涂EG和导电聚合物(如聚(3, 4-亚乙基二氧基噻吩):聚苯乙烯磺酸盐(PEDOT:PSS))的混合油墨,可以制造出表面电阻为500 Ω/sq(透光率为80%)的大面积薄膜电极。利用其优异的力学性能,这些薄膜电极已成功地集成到超薄有机光电探测器中,其性能与最先进的基于Si的无机光电探测器相当[66]。另一方面,将氧化程度相对较高的EG沉积在ITO基质上,作为检测限低、灵敏度高、可重复利用性和稳定性好的核酸传感器,EG显示出良好的性能[67]。这些应用研究不仅有助于电化学法在制备石墨烯方面的发展,也拓宽了所得石墨烯在能源、环境、生物医药等众多领域的应用。表 1总结了石墨阳极氧化所制备石墨烯的相应特征。石墨阳极氧化虽然可高效制备石墨烯,但水电解反应产生自由基发生氧化作用的同时,破坏了石墨烯六元环晶格结构和π键,需要进行还原处理恢复石墨烯原有的对称结构和性能。
| Raw material | Electrolyte | Working bias | Yield of graphene | Characteristics of graphene | ID/IG (Raman) |
C/O ratio |
Production rate | Stable graphene concentration | Ref. |
| Graphite flake or HOPG(a) | 2.25%H2SO4+ 30%KOH pH 1.2 |
(1)+2.5 V, 1 min (2) Switching (+10 V, 2 s; -10 V, 5 s) |
5%-8% | Thick < 3 nm (< 2 nm 65%) Size 1-40 μm |
0.5-1.0 | 0.085 mg·mL-1 | [30] | ||
| Graphite flakes | 0.1 mol/L H2SO4 | +10 V, 10 min | 60% | Layers 1-3 (65%) |
12.3 | [31] | |||
| Graphite flakes | 0.1 mol/L sulfuric salts (b) pH 6.5-7.0 |
+10 V, 10 min | 75% | Layers 1-3 (85%) |
0.25 | 17.2 | 16.3 g·h-1 |
0.085 mg·mL-1 in DMF |
[32] |
| Graphite foil | H2SO4, Na2SO4, LiClO4 |
(1)+2 V, 3 min (2)+10 V |
Layers 6-8 Thick 8 nm Size 3-10 μm |
1.34-0.95 (c) | 4.0-8.8 | [33] | |||
| Graphite rods | Molten salts(d) | +3 V, 3 min | 76% | Layers 1-5 Thick 0.6-2.1 nm |
0.085 mg·mL-1 in DMF | [35] | |||
| Bulk graphite and powder | Mixture of melamine and sulfuric acid | ±20 V, 10 min current 3 A | >80% | Layers < 3 (80%) |
< 0.45 | 26.2 | 1.54 g·h-1 |
0.2 mg·mL-1 |
[38] |
| Graphite rods | NaOH/H2O2/H2O | (1)+1 V, 10 min (2)+3 V, 10 min |
>70% | Layers 3-6 (95%) |
0.67 | 17.2 | [39] | ||
| Graphite foil | KOH/H2SO4/H2O pH 1.2 |
+10 V, 10 min RT 80 ℃ | 17%-77% | Layers 3-few Thick 2.4 nm | 0.33 | 1.24 | [40] | ||
| Graphite rods | 1 mol/L H2SO4:HNO3(3:1) | 3 V, 2 h | 0%-55% | Layers 2 (93%) Thick 1.0 nm |
0.345 | [42] | |||
| Graphite rods | Sodium saccharin | +10 V, 10 h | Thick 2.7 -4.0 nm | 1.12 | 57.07 mg·mL-1 |
[43] | |||
| Graphite foil | NaOH (0.8 g) PTA(e) (0.83 g) DI water 100 mL |
+10 V, 6-8 h | 99% | Layers < 6 (< 3, 78.94%) Size 1-50 μm |
3.15 | 2.5 mg·mL-1 in water |
[46] | ||
| Graphite foil | 0.1 mol/L (NH4)2SO4 addition 0.1% TEMPO(f) |
+10 V, < 10 s | Size 5-10 μm | < 0.1 | 25.3 | 15.1 g·h-1 |
6.0 mg·mL-1 in DMF |
[48] | |
| Graphite rods | 1.06 g (NH4)2SO4 +10 mL H2O2 in 80 mL water |
+10 V, 2 h 95 ℃ | Layers 2 | 0.38 | [49] | ||||
| Graphite rods | Glycine-bisulfate solution | (1)+1 V, 5 min (2)+3 V, 5 min |
Layers 2-5 < 3 layers (60%) |
0.7 | 89/11 | 0.18 g·h-1 |
2.2 mg·mL-1 |
[50] | |
| Graphite foil | Oxone (KHSO5·0.5KHSO4·0.5 K2SO4) | +50 V, 4 min | 60.1% | Layers 2-5 Size 1-5.0 μm | 1.24 | 1.04 mg·mL-1 | [51] | ||
| Natural graphite | 2 mol/L H2SO4 T 300-333 K |
CC 0.1 A, 5 min and 0.5 A, 10 min CV 1 V, 5 min and then 5 V, 10 min | Layers 2-6(CC) 2-5(CV) |
CC>0.25 CV>0.29 |
[52] | ||||
| Expandable graphite | KOH/H2SO4 | +15V, 0.11 A·cm-2 | Layers 6 Thick 2-3 nm Size 2.85 μm |
0.061 | 8.65 | [55] | |||
| Graphite sheets | NaOH+Na2S2O3+NaClO4 | 10 V, 3 h | Layers 4-5 Thick < 3 nm Size 4 μm |
0.35 | 27.7 | [57] | |||
| Graphite sheets | 0.1, 0.3, 0.5, 1.0 mol/L H2SO4 |
0-10 V | Layers 4-6 Size 11-26 μm |
1.0 | [58] | ||||
| HOPG | HClO4 | 0-8 V | 40%-44% | Layers 3-6 | 0.96- 1.04 |
1.6 | [59] | ||
| Graphite flakes | 8 mol/L HClO4 | 1.2-1.4 V | 0.16-0.75 | 43.2- 9.81 |
[60] | ||||
| Flexible graphite paper | Step 1 98% H2SO4 Step 2 50% H2SO4 |
Step 1+1.6 V, 20 min Step 2+5 V, < 2 min |
96% | Layer 1 (95%) Layers 2(5%) Size < 1 μm |
0.16-0.75 | 1.5-1.8 | 12 g·h-1 | 2.0 mg·mL-1 | [61] |
| Note:(a)HOPG: highly ordered pyrolytic graphite; (b)sulfate salts: sodium/potassium/ammonium sulfate; (c)ID/IG:H2SO41.34, Na2SO4 0.95, LiClO4 1.0;C/O:H2SO4 8.1, Na2SO4 8.8, LiClO4 4.0;(d)molten salts:acetamide, urea and ammonium nitrate; (e)PTA:p-phthalic acid; (f)TEMPO:((2, 2, 6, 6-tetramethylpiperidin-1-yl)oxyl). | |||||||||
受碳酸丙烯酯(PC)在锂离子电池循环过程中破坏行为的启发,2011年,研究者以LiClO4的PC溶剂为电解液,在电场作用下,阴极HOPG表面形成的[Li·2PC]+和[Li·3PC]+阳离子共同嵌入石墨层间而促使石墨膨胀,继而在LiCl/N, N-二甲基甲酰胺(DMF)/PC混合液中借助于超声波作用阴极石墨被剥离成分散性好、导电性优、产率高达70%的少层(< 5)高质量石墨烯[68]。当研究者以NaClO4的乙腈(ACN)溶剂为电解质,通过施加+5 V电压作用30 min,可将较大的ClO4-嵌入到石墨层中,当它穿过石墨阳极晶界或缺陷位置时,较小的、不带电荷的ACN分子会随之渗透到石墨层中,然后用微波辐照通过ACN分解和气体膨胀完成石墨烯剥离过程。所得石墨烯的产率为61%,其中单层占28%,双层占69%,平均横向尺寸为1~2 μm,并且对比实验还发现,在仅使用ClO4-而无ACN的情况下,未观察到插层现象发生[69]。Zhou等[70]报道以NaCl和二甲基亚砜(DMSO)混合液作为电解质,巯基乙酸作为添加剂,Na+常与4~5个DMSO分子形成溶剂化配合物,然后插入石墨层间形成三元石墨插层化合物(Na+(DMSO)yCn-)。该化合物层间距为1.246 nm,几乎是石墨层距(0.335 nm)的4倍,因此会产生巨大内应力而使阴极石墨膨胀剥落,再经温和超声波可剥离成具有高导电性的少层石墨烯,同时产物分子中芳香环原位结合巯基乙酸中两个亲水—NH2达到稳定。总之,石墨阴极剥离过程中,借助于电解质阳离子与溶剂分子间的协同作用膨胀石墨间层减弱范德华力,可提高石墨烯剥离产率和效率。
阴极石墨插层剥离制备石墨烯的产率受溶剂类型的影响,在含四烷基铵阳离子的电解质中,如分别以四丁基六氟磷酸铵(TBAPF6)的DMF,ACN和PC溶剂为电解质时,溶剂化阳离子TBA+插入石墨层间形成的插层化合物((solv)mTBA+Cn-)会促进溶剂分子插入,进而使石墨膨胀和层间范德华力减弱,最终可剥离成晶格间距为0.34 nm、由3~6层组成的GNs[71]。对比实验发现,在ACN溶剂中,石墨剥落速率最快(0.5 h可产生明显气泡),但所得GNs较在DMF和PC溶剂中要厚,说明溶剂不同会影响石墨插层剥离的效率[71]。阴极石墨插层剥离制备石墨烯的产率还受阳离子直径大小影响,在含四辛基溴化铵(TOAB)的DMF电解液中进行阴极HOPG插层剥离时,阳离子TOA+与石墨层间距(0.335 nm)相比尺寸较大(2 nm),插入石墨层间后会导致层间范德华力不可逆断裂,在阴极电化学还原和超声波共同作用下使石墨最终剥离成石墨烯[72];以四甲基高氯酸铵(TMAClO4)的NMP为电解质,阳离子(TMA+)插层也可使阴极石墨剥离得到石墨烯[73];在含阳离子(四丙基铵和己基三甲基铵)的电解质溶液中,对一些预膨胀边缘和含层间空隙(如石墨箔)的阴极石墨剥离制备石墨烯的产率高达40%~50%[74]。综合比较上述研究结果,TBA+(直径0.826 nm)比四乙基铵(TEA+) (直径0.674 nm)、TMA+(直径0.558 nm)能更有效地剥离HOPG阴极[75],这主要是由于较大铵离子能被压扁插入到石墨层间,比较小的铵离子更能有效膨胀石墨。因此,在阴极石墨插层中,选择合适的阳离子、溶剂及石墨电极均可提高阴极剥离的效率和产率。
离子液体(ILs)被认为是传统有机电解质的替代品。早期研究表明,咪唑基离子液体中含少量水对石墨有效剥离至关重要。如在1-丁基-3-甲基咪唑硼酸盐(BMIMBF4)离子液体中电解石墨棒时,BMIM+在无水环境中不能插入石墨层间[76];但是,在以质子型1-丁基-3-甲基咪唑硫酸氢盐(BMIMHSO4)为电解质的电解液中,当H+在阴极被还原成H2时,可有效地打开阴极石墨层末端,进而促进较大BMIM+离子插入石墨层间[77]。之后,随着H+和BMIM+插入量的增加,咪唑环和碳原子之间的剪切力和π-π相互作用,使石墨层被BMIM+有效地剥落,并在机械研磨剪切力作用下剥离成少层石墨烯,其中,在阴极处水电解产生的H+是促使BMIM+嵌入石墨层间的关键因素。与传统的水基电解质体系相比,ILs/ACN电解质(体积比1:50)中单层石墨烯的产率(4 h, 86%)更高。但是,ILs分子在石墨表面上的电化学反应会改变石墨烯性能,消除这些官能团需要进行后处理,这又使其成本较高、应用受到限制[78]。
3.2 形貌结构为了进一步提高阴极石墨膨胀效果获得较大尺寸的石墨烯,2012年,研究者提出了一种先插层较小阳离子、再嵌入较大阳离子的两步法[79]。首先将石墨箔片在含LiClO4/PC的电解液中进行预处理插入Li+/PC配合物,然后再将四丁基高氯酸铵(TBAClO4)加入到电解液中,较大TBA+渗透嵌入到石墨层间并与较小Li+交换,促使石墨膨胀范德华力减弱而剥落,最后经温和超声波可获得产率在30%~40%之间、尺寸约为20 μm的石墨烯[70]。但由于TBA+的插层时间较长(24 h),影响进一步放大应用。
DMSO具有较宽的电化学窗口,其表面能(43.5 mJ/m2)接近于石墨表面能(53 mJ/m2)[80],可有效分散石墨烯,因此常用作制备石墨烯的溶剂。以盐酸三乙基胺(Et3NHCl)和LiClO4的DMSO溶液为电解质,由于DMSO的溶剂化作用,Li+,Et3NH+两种离子与DMSO共嵌入石墨层间后,原位形成的配合物会分解生成SO2气体并削弱其层间的范德华力,经剥离最终可获得大尺寸(< 20 μm)、厚度小于7层的石墨烯[81]。此外,在1 mol/L LiCl-DMSO电解质中阴极石墨也可剥离成尺寸范围为1~20 μm、厚度小于5 nm(其中5%小于0.9 nm)的石墨烯[82]。
3.3 质量和缺陷密度石墨阴极插层剥离通常可制备高质量和低缺陷密度的石墨烯,如在LiClO4/PC的电解液中,施加高电压((-15±5) V)来驱动Li+/PC共嵌入阴极石墨中,经剥离和超声分散后,最终所得少层石墨烯的ID/IG比值低于0.1,这与氧化石墨烯相比其缺陷密度低得多[68];以高氯酸钠(NaClO4)的ACN溶液为电解质时,所得石墨烯的产率为61%,其中,ID/IG强度比值为1.87,C/O仅为0.83[69];以四烷基铵阳离子为插层剂,电解石墨阴极获得石墨烯与石墨前体相比含氧量仅增加了3%[71]。因此,通过电化学剥离阴极石墨可制备高质量的石墨烯。
石墨阴极插层剥离石墨烯的效率、产率、形貌、质量及缺陷密度与阳离子类型、溶剂和插层电压有关,选择合适的插层剥离条件,通过超声波辅助剥离后,可得到尺寸低于20 μm、产率最高可达60%的层状高质量石墨烯。表 2总结了石墨阴极插层所制备石墨烯的相应特征。研究者将其用在储能材料中,显示出良好的性能,如用2% EG改性的磷酸铁锂,其比电容量可达208 mAh/g,这是由于包裹在磷酸铁锂周围的高导电石墨烯薄片有助于充电/放电过程中的电子迁移,并可产生约100%的库仑效率[83]。基于EG制备的石墨烯电容器可逆循环30次后电容量仍达388 mAh/g,明显高于天然石墨阳极的电容量(240 mAh/g)[84]。同样,借助于电解质锂离子和剥落的石墨烯薄片之间的可逆氧化-还原反应,EG与少量氧化钴的纳米复合材料,也具有良好的电容电荷存储特性[85]。由于在插层过程中没有强氧化自由基产生,石墨阴极插层剥离法避免了石墨烯的过度氧化,但得到的石墨烯常为多层结构,并且插层时间较长,常需几十个小时,且所用有机溶剂大多具有毒性、离子液体价格也较高,这使得该法应用受到限制。
Raw material |
Electrolyte | Working bias | Yield of graphene | Characteristics of graphene | ID/IG (Raman) |
C/O ratio |
Production rate | Stable graphene concentration | Ref. |
| Graphite flakes | 30 mg·mL-1 LiClO4 in PC(a) |
(-15±5) V, 2 min | 70% | Layers < 5 | < 0.1 | 0.12 g·h-1 |
10 mg·mL-1 in DCB |
[68] | |
| Graphite rods | NaClO4 in AN(b) | 0-5 V, 30 min | 61% | Layers Single (28%) Double (69%) |
0.79 after reduction |
12.7 | 0.24 mg·mL-1 in DMF(c) |
[69] | |
| Graphite flake or HOPG | Mixture of NaCl, thionin acetate salt in DMSO(d) | -5 V | Layers 7-8 Thick 3.1 nm |
0.1 | 12.5 | [70] | |||
| HOPG/graphite rod | TABPF6+LiPF6(e) in AN/PC/DMF |
-10 V, 0.2 mA | Layers 3-6 | [71] | |||||
| HOPG | TOA+, TMA+, TEA+(f) |
-10 V, 3 h -1 V, 2 h |
70% | Layers < 3 (70%) Thick 2-3.8 nm |
< 3% | 17.5 μg·mL-1 in DMF |
[72] | ||
| Graphite foil/rod HOPG | TPA+, HTMA+(g) | -10 V, 2 h | 40%-50% | 0.25 mg·mL-1 |
[74] | ||||
| Graphite foil | 0.01 mol/L AgClO4 and 0.1 mol/L TBAP(h) in NMP |
CV scan from 0 to -6 V | Thick 2 nm | 15.3 | 0.01 mg·mL-1 in NMP |
[75] | |||
| HOPG | BMIMHSO4(i) | 3.0 V | Layers 1-3 | 0.19 0.04 |
[77] | ||||
| Graphite rod | 0.1 mol/L IL(j)acetonitrile |
7.0 V, 4 h | 29%-86% | Layers 1-5 | 1 mg·mL-1 in NMP |
[78] | |||
| Natural graphite flakes | LiClO4+TBAP in PC |
(1)-3 V to -5 V, 5 min; (2)-5 V, 24 h | 30%-40% | 0.1 mg·mL-1 in DMF |
[79] | ||||
| Natural graphite flake or HOPG | Mixture of LiCl, Et3NHCl (k) in DMSO |
CV scan from 0 to -6 V | Layers < 7 Size < 20 μm |
0.3 | 15.7 | 0.5-2 g·h-1 |
[81-82] | ||
| Note:(a)PC:propylene carbonate; (b)AN:acetonitrile; (c)DMF:N, N-dimethylformamide; (d)DMSO:dimethyl sulfoxide; (e)TABPF6:tetrabutylammonium hexafluorophosphate, LiPF6:lithium hexafluorophosphate; (f)TOA+:tetra-n-octylammonium, TMA+or TEA+:tetramethyl or tetraethyl ammonium ions; (g)TPA+:tetrapropylammonium, HTMA+:hexyltrimethylammonium; (h)TBAP:tetra-n-butylammonium perchlorate; (i)BMIMHSO4:1-butyl-3-methyl imidazoliumbisulfate; (j)IL:EMIMBF4, 1-ethyl-3-methylimidazolium tetrafluoroborate; EMIM BTA, 1-ethyl-2-methylimidazolium-bis-(trifluoromethyl)sulfonyl)amide, BMPyrr BTA, 1-butyl-1-methylpyrrolidinium-bis-(trifluoromethyl)sulfonyl)amide; (k)Et3NHCl: triethylamine hydrochloride. | |||||||||
电化学法剥离石墨电极常借助于机械和超声波作用力以提高制备石墨烯的效率和质量。Shinde等[86]报道了采用自制的电化学微流控反应器(图 4所示),通过硫酸盐电解质离子流动产生的诱导剪切力引发石墨剥落,获得的石墨烯尺寸约为10 μm(>75%低于4层),ID/IG比值仅为0.21~0.32,这说明流体动力学力的辅助作用可强化石墨烯的剥离效率和质量。Afkham等首先采用电化学法将阳极HOPG插层,形成相邻层之间具有强弱交替吸引力的GIC中间体,之后借助于可调强度的超声剪切力,使GIC在弱吸引力位置发生裂解,产生富含双层或三层的石墨烯薄片[87-88]。此外,借助于超声波作用力对电解质均匀化,可降低石墨烯中含氧基团的比率,从而提高石墨烯的性能[89]。
声电化学是耦合了超声辐射与电化学体系的一种作用,具有破坏扩散层、提高插层离子穿过、双电层传输能力,如研究者借助于声电化学介质十二烷基硫酸钠(SDS)在HOPG阳极上的插层剥落作用,成功制备了具有不同缺陷程度的少层石墨烯[90]。Thanh等[91]报道了利用超声能量与电化学的协同作用诱导等离子体在阴极原位放电,在中等温度下,不需要酸性介质或昂贵的离子电解质的条件下制备了厚度为2.5 nm(约7层)、尺寸为6 μm的石墨烯。
4.2 石墨形态及电解池结构电化学剥离石墨制备石墨烯的过程中,其他因素如阳极石墨形态和电解池结构也会影响石墨烯质量或剥离效率。据报道,用类似“蠕虫”状结构的膨胀石墨代替天然石墨作为阳极[92],可获得产率达75%的少层石墨烯(< 7层),且石墨烯氧含量不高于天然石墨。以光谱石墨棒(SPG)和HOPG作为阳极,制备石墨烯产率分别为76%和42%,且用SPG阳极制备石墨烯的氧含量(约15.3%)显著低于HOPG (约29.9%)[93]。Liu等[94]也报道了以铅笔芯为石墨阳极,在磷酸或硫酸水溶液中通过电解剥离制备少层氧化石墨烯的方法。Cooper等[75]报道选用HOPG作为阴极,必须在超声波辅助下才能剥离成横向尺寸较大的石墨烯片,但石墨棒电极可直接产生尺寸很小(约100~200 nm)、厚度约为2~5层的石墨烯薄片,整个过程不需要超声处理。原因是HOPG高度有序的晶体取向,单独插层电解质铵离子未能使石墨层膨胀到断裂点,因此需借助于超声作用力克服附加于层间的结合能。但石墨棒中石墨烯片处于随机取向,不存在高结晶取向产生的结合能。
此外,结构设计合理的电解池也有助于石墨的插层与剥离,如将石墨阳极置于电解池底部、惰性阴极铂放在顶部的垂直结构,剥离后薄片石墨烯悬浮在溶液中,厚重石墨颗粒在底部经反复剥离,直到石墨烯足够轻薄悬浮在溶液中,最终获得石墨烯产率约为50%(其中4~8 nm厚片,约占80%)[95]。西安交通大学徐友龙团队[96]采用电化学和热分解双石墨电极获得了高产量的少层石墨烯(图 5所示)。他们将设计的夹层石墨电极包裹在具有多孔金属网的密闭空间结构中,以实现足够多的离子嵌入,结合电化学离子插层和之后的热分解使石墨急剧膨胀,从而高效地制备了高质量的石墨烯。通过精确控制离子插层,阳极和阴极石墨烯的剥离效率分别为48%和85%,生产率超过25 g/h,石墨烯产品(< 3层高达70%)显示出优异的晶格质量(ID/IG < 0.08)和导电性(>3×104S/m)。此外,产品在N-甲基吡咯烷酮(浓度可达10 mg·mL-1)中的良好分散性和优异的加工性使它能够制造大面积高导电性(11 Ω/sq)柔性石墨烯薄膜,这为高质量石墨烯的大规模生产和广泛应用带来了希望。
为解决必须用石墨整料作为电化学法制备石墨烯的电极和因膨胀导致剥离过程无法持续进行的问题,近来,研究者将没有添加黏合剂的石墨片引入到管状透析膜(作为可渗透容器)内制成了一种可压缩和放大的工作电极,石墨箔作为对电极,同时被浸入到(NH4)2SO4电解液中,当向工作电极施加电压(+10 V)时,石墨被插层离子(SO42-)连续地嵌入、膨胀和剥离,最终获得的少层石墨烯产率达65%,横向尺寸>30 μm,厚度为2~7 nm,氧原子分数为16.7%,C/O比为4.98,ID/IG比值为0.9~1.2,这与化学或热还原GO的ID/IG比值(1.1~1.5)类似(图 6(a))。此外,除批量反应器外,作者还提出了一种连续电化学剥离方案,将上述石墨工作电极放在活塞流反应器中,电解质在压力下通过多孔管或脊状网也进入反应器中,促使石墨在反应器内不断剥落制备成石墨烯(图 6(b)),这实现了石墨粉在可渗透和扩展的容器中作为整体工作电极连续制备石墨烯的设想[97]。

|
图 6 可渗透、可膨胀容器中电化学剥离石墨(a)和具有强制电解质流动的可渗透的活塞流反应器中电化学剥离连续生产石墨烯(b)示意图[97] Fig. 6 Schematic illustration of the process for electrochemical exfoliation of graphite flakes in a permeable, expandable container(a) and continuous production of electrochemical exfoliation graphene in a permeable plug flow reactor with forced electrolyte flow(b)[97] |
如上所述,通过改变阴极或阳极电化学剥离过程中的电解质类型、外加电压、石墨形态等条件可制备具有不同产率、形貌、质量和缺陷的石墨烯,以满足各种应用的需求。在实际应用中,石墨烯由于其比表面积巨大易于堆叠,使它优异的结构特征和性能常常无法真正显现和利用。因此,需要对其进行功能化处理,制备成功能化石墨烯材料(FG),以避免片层间重新堆叠和提高其应用性能。
目前,电化学法制备功能化石墨烯(EFG)材料主要是通过化学修饰和化学掺杂两种途径实现的,除了在传感器和复合材料中应用外,目前大多应用于储能和能量转换领域,尤其是应用在超级电容器或微型超级电容器(SC或MSC)中。化学修饰方面,在NaOH存在条件下,以磺化聚醚醚酮(PEEK)水溶液为电解质和表面改性剂,可直接由石墨棒经电化学法一步合成产率达40%、比表面积为433 m2/g的FG,且在电流密度为2.2 A/g的条件下,以FG作为电极的比电容量为244 F/g[98]。另外,石墨经电解法插层和聚合丙烯腈分子形成的聚丙烯腈(PAN)/FG作为阳极时,其初始放电容量超过2000 mAh/g,可逆电容量约为300 mAh/g[99];将采用一步电化学法制备包含铁、钴和钒氧化物掺杂的石墨烯基纳米复合材料,其中负载Fe2O3复合材料作为阳极时,其电容量高达894 mAh/g[83];将电化学剥离石墨过程中结合原位电沉积硫制备的石墨烯-硫复合材料作为锂-硫电池阴极材料时,在0.1 A/g条件下其初始放电容量为1080 mAh/g,经过60个循环周期后,其电容量仍保持在900 mAh/g以上[100]。总之,采用电化学法通过化学修饰途径制备的FG用作为储能电极材料时,具有优良的充放电性能。
在化学掺杂方面,电化学法制备的氮掺杂功能化石墨烯材料(NFG)可以调节电子结构、改善材料物理与化学性能,进而促进了自身的实际应用。其中含氮电解质的选择是影响NFG的酰胺化水平和氨基分布的关键因素,低电位剥离有助于原位氨基官能化反应,使NFG表面含有较多的氮。研究者在含氮电解质(NH4)2HPO4,(NH4)2SO4,NH4NO3水溶液中,采用一步电化学法从HOPG电极剥离制备了原位不同氮掺杂(酰胺化)的石墨烯[101];在3 mol/L甘氨酸和氨(25%~28%)的混合溶液中,也可实现氮掺杂多层石墨烯片(N-FLGS)的阳极电化学制备,其过程包括甘氨酸阴离子H2NCH2COO-插层进入石墨层、氧化产物H2NCH2COHCH2—的电聚合以及石墨层的原位氮掺杂三个过程,制备的产物中氮掺杂量高达6.05%(原子分数),将其作为超级电容器电极材料时显示出优异的循环稳定性(10000次循环后的电容率为96.1%)和显著的快速充放电能力(1 A/g时为184.5 F/g,50 A/g时为132.7 F/g)[102]。
除了制备氮掺杂的FG外,电化学法还可用于制备具有优异性能的氧、磷、氟掺杂的FG材料,如在0.03 mol/L植酸(PA)和0.1 mol/L (NH4)2SO4组成的混合电解质中,采用电化学法阳极剥离石墨箔制备了氧、磷掺杂的功能化多孔石墨烯(FHG)。进一步可制造出具有较大离子可及表面积、有效电子和离子输送路径的FHG-MSC,其区域电容随电极厚度线性增大,面积电容可达6.41 mF/cm2,体积电容可达30.51 F/cm3,能量密度可高达4.24 mWh/cm3;此外,FHG-MSC在500个弯曲周期内具有良好的机械柔韧性,电容保持率达99.7%[103]。近来,中国科学院大连化学物理研究所吴忠帅团队[104]在环境友好的中性水系四氟硼酸钠(NaBF4)电解液中,采用电化学法剥离石墨制备了横向尺寸达12 μm、厚度小于3层、产率高达70%的氟掺杂石墨烯(FFG),进一步组装的高比能全固态微型石墨烯超级电容器(FFG-MSC),其能量密度高达59 mWh/cm3。同时,FFG-MSC具有优异的柔性和循环稳定性,在180°弯曲时具有100%的电容保持能力,在弯曲状态下5000次循环后容量保持率为93%[104]。此外,这种微型储能器件还表现出良好的模块化集成能力,可有效调控输出工作电压和容量,这为大规模、高效制备功能化石墨烯及高性能柔性超级电容器铺平了道路。
6 结束语电化学法插层剥离石墨是一种经济有效、可规模化的石墨烯或功能化石墨烯制备方法,其发展能促进石墨烯在电子器件、储能转化、催化、传感器等领域广泛应用。研究者针对石墨烯的剥离产率、形貌、质量及缺陷密度、工艺开发等进行了大量研究,虽然取得了一些阶段性成果,但还存在一些问题需解决。
(1) 电解质的设计
综上研究结果表明,石墨烯的产率、形貌、质量及缺陷密度主要取决于电解质的选择和插层条件的控制。在含水电解质中阳极剥离有利于形成少层石墨烯(1~3层),但水电解产生自由基的强氧化性会导致石墨烯sp2结构被破坏。理想的电解质体系不仅能诱导离子有效嵌入和剥离石墨烯层且不会破坏石墨形态,还可以作为分散剂稳定石墨烯片,以避免重新聚集。在有机溶剂中阴极阳离子插层虽然可避免石墨烯过度氧化,但获得少层石墨烯的产率通常较低,因此,在阳离子插层过程中,在电解质中引入气态物质膨胀石墨层有助于实现高质量石墨烯的制备。
(2) 剥离机理的研究
由于电化学反应的复杂性,石墨界面的膨胀剥离过程也较复杂。从机理上解释一个成功的剥离过程包括:阴离子/阳离子插入、石墨层膨胀和表面石墨烯片剥落3个步骤。通过监测石墨界面的结构形变,Palermo等[105]证明了石墨的有效插层和气泡的析出膨胀是剥离快速完成的关键。实际上,石墨电极的任何部位均会膨胀剥落,并且剥离不是有序发生的,这是造成石墨烯剥离后层数分布较广的主要原因,因此在实际操作中需要根据应用进一步权衡石墨烯剥离的效率和质量。总之,深入理解电化学剥离机理对优化制备工艺、获得尺寸和厚度分布均匀的石墨烯至关重要。
(3) 剥离条件的优化
除电解质外,石墨原料对剥离石墨烯的形貌、氧含量和缺陷密度也有很大的影响。研究者已探索了各种石墨前体的电化学剥离过程[106],如HOPG、膨胀石墨、天然石墨片、石墨粉、石墨箔和石墨插层化合物等,结果证明,根据不同电化学电极的需要合理选择起始石墨材料是获得高质量石墨烯片的关键。同时,施加在石墨上的电位变化可能是控制石墨烯剥离效率、层数和质量的另一个重要因素[107]。
(4) 电解池设备设计
为了使电化学制备石墨烯方法从实验室转化到工业化生产,电解池的合理设计至关重要,尤其是实现电化学的连续生产和产物分离,如电解质的循环利用及去除未剥离的杂质等,这也是扩大生产规模的重要条件。
| [1] |
崔超婕, 田佳瑞, 杨周飞, 等. 石墨烯在锂离子电池和超级电容器中的应用展望[J]. 材料工程, 2019, 47(5): 1-9. CUI C J, TIAN J R, YANG Z F, et al. Application prospect of graphene in Li-ion battery and supercapacitor[J]. Journal of Materials Engineering, 2019, 47(5): 1-9. |
| [2] |
NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669. |
| [3] |
SONG N, JIA J, WANG W, et al. Green production of pristine graphene using fluid dynamic force in supercritical CO2[J]. Chemical Engineering Journal, 2016, 298(8): 198-205. |
| [4] |
PATON K R, VARRLA E, BACKES C, et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids[J]. Nature Materials, 2014, 13(6): 624-630. |
| [5] |
MARCANO D C, KOSYNKIN D V, BERLIN J M, et al. Improved synthesis of graphene oxide[J]. ACS Nano, 2010, 4(8): 4806-4814. |
| [6] |
JOSHIA R K, ALWARAPPAN S, YOSHIMURA M, et al. Graphene oxide:the new membrane material[J]. Applied Materials Today, 2015, 1(1): 1-12. |
| [7] |
DESILVA K K H, HUANG H H, JOSHI R K, et al. Chemical reduction of graphene oxide using green reductants[J]. Carbon, 2017, 119(8): 190-199. |
| [8] |
TALYZIN A V, MERCIER G, KLECHIKOV A, et al. Brodie vs Hummers graphite oxides for preparation of multi-layered materials[J]. Carbon, 2017, 115(5): 430-440. |
| [9] |
ALAM S N, SHARMA N, KUMAR L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO)[J]. Graphene, 2017, 6(1): 1-18. |
| [10] |
XIN H, ZHAO Q, CHEN D, et al. Roll-to-roll mechanical peeling for dry transfer of chemical vapor deposition graphene[J]. Journal of Micro and Nano-Manufacturing, 2018, 6(3): 031004-031011. |
| [11] |
XIN H, LI W. A review on high throughput roll-to-roll manufacturing of chemical vapor deposition graphene[J]. Applied Physics Reviews, 2018, 5(3): 031105-031121. |
| [12] |
CHEN Z, QI Y, CHEN X, et al. Direct CVD growth of graphene on traditional glass:methods and mechanisms[J]. Advanced Materials, 2018, 31(9): 1803639. |
| [13] |
SUN J, CHEN Z, YUAN L, et al. Direct-chemical-vapor-deposition-fabricated, large-scale graphene glass with high carrier mobility and uniformity for touch panel applications[J]. ACS Nano, 2016, 10(12): 11136-11144. |
| [14] |
CHEN X, CHEN Z, JIANG W, et al. Fast growth and broad applications of 25-inch uniform graphene glass[J]. Advanced Materials, 2017, 29(1): 1603428-1603434. |
| [15] |
CIESIELSKI A, SAMORÌ P. Graphene via sonication assisted liquid-phase exfoliation[J]. Chemical Society Reviews, 2014, 43(1): 381-398. |
| [16] |
HAAR S, EL GEMAYEL M, SHIN Y, et al. Enhancing the liquid-phase exfoliation of graphene in organic solvents upon addition of n-octylbenzene[J]. Scientific Reports, 2015, 5: 16684-16693. |
| [17] |
DÖBBELIN M, CIESIELSKI A, HAAR S, et al. Light-enhanced liquid-phase exfoliation and current photoswitching in graphene-azobenzene composites[J]. Nature Communications, 2016, 7(7): 11090-11099. |
| [18] |
SÉBASTIEN H, ARTUR C, JOSEPH C, et al. A supramolecular strategy to leverage the liquid-phase exfoliation of graphene in the presence of surfactants:unraveling the role of the length of fatty acids[J]. Small, 2015, 11(14): 1691-1702. |
| [19] |
CONTI S, DEL ROSSO M G, CIESIELSKI A, et al. Perchlorination of coronene enhances its propensity for self assembly on graphene[J]. ChemPhysChem, 2016, 17(3): 352-357. |
| [20] |
ARTUR C, SéBASTIEN H, MIRELLA E G, et al. Harnessing the liquid-phase exfoliation of graphene using aliphatic compounds:a supramolecular approach[J]. Angewandte Chemie International Edition, 2014, 53(39): 10355-10361. |
| [21] |
王晨, 燕绍九, 南文争, 等. 高浓度石墨烯水分散液的制备与表征[J]. 材料工程, 2019, 47(4): 56-63. WANG C, YAN S J, NAN W Z, et al. Preparation and characterization of high concentration graphene aqueous dispersion[J]. Journal of Materials Engineering, 2019, 47(4): 56-63. |
| [22] |
YANG S, ZHANG P, NIA A S, et al. Emerging 2D materials produced via electrochemistry[J]. Advanced Materials, 2020, 32(10): 1907857. DOI:10.1002/adma.201907857 |
| [23] |
AMBROSI A, PUMERA M. Exfoliation of layered materials using electrochemistry[J]. Chemical Society Reviews, 2018, 47: 7213-7224. |
| [24] |
平蕴杰, 龚佑宁, 潘春旭. 电化学剥离制备石墨烯及其光电特性研究进展[J]. 中国激光, 2017, 44(7): 98-113. PING Y J, GONG Y N, PAN C X. Research progress in preparation of graphene from electrochemical exfoliation and its optoelectronic characteristics[J]. Chinese Journal of Lasers, 2017, 44(7): 98-113. |
| [25] |
KURYS Y I, USTAVYTSKA O O, KOSHECHKO V G, et al. Structure and electrochemical properties of multilayer graphene prepared by electrochemical exfoliation of graphite in the presence of benzoate ions[J]. RSC Advances, 2016, 6(42): 36050-36057. |
| [26] |
ABDELKADER A M, COOPER A J, DRYFE R A, et al. How to get between the sheets:a review of recent works on the electrochemical exfoliation of graphene materials from bulk graphite[J]. Nanoscale, 2015, 7(16): 6944-6956. |
| [27] |
SINGH R, CHARU TRIPATHI C. Synthesis of colloidal graphene by electrochemical exfoliation of graphite in lithium sulphate[J]. Materials Today:Proceedings, 2018, 5(1): 973-979. |
| [28] |
YU P, SIMON G, ZHONG Y. Electrochemical exfoliation of graphite and production of functional graphene[J]. Current Opinion in Colloid & Interface Science, 2015, 20(5/6): 329-338. |
| [29] |
刘琳, 李莹, 鄂涛, 等. 球状纳米二氧化钛/石墨烯复合材料的合成及导电性能[J]. 材料工程, 2019, 47(8): 97-102. LIU L, LI Y, E T, et al. Synthesis and electrical conductivity of spherical nano-TiO2/graphene composites[J]. Journal of Materials Engineering, 2019, 47(8): 97-102. |
| [30] |
SU C Y, LU A Y, XU Y, et al. High-quality thin graphene films from fast electrochemical exfoliation[J]. ACS Nano, 2011, 5(3): 2332-2339. |
| [31] |
PARVEZ K, LI R, PUNIREDD S R, et al. Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics[J]. ACS Nano, 2013, 7(4): 3598-3606. |
| [32] |
PARVEZ K, WU Z S, LI R, et al. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts[J]. Journal of the American Chemical Society, 2014, 136(16): 6083-6091. |
| [33] |
SONG Y, XU J L, LIU X X. Electrochemical anchoring of dual doping polypyrrole on graphene sheets partially exfoliated from graphite foil for high-performance supercapacitor electrode[J]. Journal of Power Sources, 2014, 249(1): 48-58. |
| [34] |
ZHANG Y, XU Y L, ZHU J B, et al. Electrochemically exfoliated high-yield graphene in ambient temperature molten salts and its application for flexible solid-state supercapacitors[J]. Carbon, 2018, 127(2): 392-403. |
| [35] |
AMBROSI A, PUMERA M. Electrochemically exfoliated graphene and graphene oxide for energy storage and electrochemistry applications[J]. Chemistry-A European Journal, 2016, 22(1): 153-159. |
| [36] |
HAMRA A A B, LIM H N, CHEE W K, et al. Electro-exfoliating graphene from graphite for direct fabrication of supercapacitor[J]. Applied Surface Science, 2016, 360(1): 213-223. |
| [37] |
RADON A, WŁODARCZYK P, ŁUKOWIEC D. Structure, temperature and frequency dependent electrical conductivity of oxidized and reduced electrochemically exfoliated graphite[J]. Physica E, 2018, 99: 82-90. |
| [38] |
CHEN C H, YANG S W, CHUANG M C, et al. Towards the continuous production of high crystallinity graphene via electrochemical exfoliation with molecular in situ encapsulation[J]. Nanoscale, 2015, 7(37): 15362-15373. |
| [39] |
RAO K S, SENTHILNATHAN J, LIU Y F, et al. Role of peroxide ions in formation of graphene nanosheets by electrochemical exfoliation of graphite[J]. Scientific Reports, 2014, 4(6174): 1032-1035. |
| [40] |
TRIPATHI P, PRAKASHPATEL C R, DIXIT A, et al. High yield synthesis of electrolyte heating assisted electrochemically exfoliated graphene for electromagnetic interference shielding applications[J]. RSC Advances, 2015, 5(25): 19074-19081. |
| [41] |
LIU Z, LIU J Z, YAO C, et al. Interlayer binding energy of graphite:a mesoscopic determination from deformation[J]. Physical Review B, 2012, 85(20): 205418-23. |
| [42] |
COROS M, POGACEAN F, ROSU M C, et al. Simple and cost-effective synthesis of graphene by electrochemical exfoliation of graphite rods[J]. RSC Advances, 2016, 6(4): 2651-2661. |
| [43] |
KUMAR M K P, SHANTHINI S, SRIVASTAVA S. Electrochemical exfoliation of graphite for producing graphene using saccharin[J]. RSC Advances, 2015, 5(66): 53865-53869. |
| [44] |
YANG S, LOHE M R, MVLLEN KLAUS, et al. New-generation graphene from electrochemical approaches:production and applications[J]. Advanced Materials, 2016, 28(29): 6213-6221. |
| [45] |
钟轶良.电化学剥离制备石墨烯及其石墨烯用作燃料电池催化剂载体的研究[D].广州: 华南理工大学, 2013. ZHONG Y L. Investigation for the preparation of graphene with electrochemical exfoliation and the application of graphene as support for fuel cell catalyst[D].Guangzhou: South China University of Technology, 2013. |
| [46] |
TANG H, HE P, HUANG T, et al. Electrochemical method for large size and few-layered water-dispersible graphene[J]. Carbon, 2019, 143(3): 559-563. |
| [47] |
WANG H S, TIAN S Y, YANG S W, et al. Anode coverage for enhanced electrochemical oxidation:a green and efficient strategy towards water-dispersible graphene[J]. Green Chemistry, 2018, 20(6): 1306-1315. |
| [48] |
YANG S, BRVLLER S, WU Z S, et al. Organic radical assisted electrochemical exfoliation for the scalable production of high-quality graphene[J]. Journal of the American Chemical Society, 2015, 137(43): 13927-13932. |
| [49] |
HOSSAIN S T, WANG R. Electrochemical exfoliation of graphite:effect of temperature and hydrogen peroxide addition[J]. Electrochimica Acta, 2016, 216(10): 253-260. |
| [50] |
RAO K S, SENTILNATHAN J, CHO H W, et al. Soft processing of graphene nanosheets by glycinebisulfate ionic-complex-assisted electrochemical exfoliation of graphite for reduction catalysis[J]. Advanced Functional Materials, 2015, 25(2): 298-305. DOI:10.1002/adfm.201402621 |
| [51] |
TIAN S, YANG S, HUANG T, et al. One-step fast electrochemical fabrication of water-dispersible graphene[J]. Carbon, 2017, 111(1): 617-621. |
| [52] |
HSIEH C T, HSUEH J H. Electrochemical exfoliation of graphene sheets from a natural graphite flask in the presence of sulfate ions at different temperatures[J]. RSC Advances, 2016, 6(69): 64826-6483. |
| [53] |
HSIEH C T, YANG B H, CHEN Y F. Dye-sensitized solar cells equipped with graphene-based counter electrodes with different oxidation levels[J]. Diamond & Related Materials, 2012, 27/28(7): 68-75. |
| [54] |
HSIEH C T, YANG B H, LIN J Y. One-and two-dimensional carbon nanomaterials as counter electrodes for dye-sensitized solar cells[J]. Carbon, 2011, 49(9): 3092-3097. |
| [55] |
CHUANG C H, SU C Y, HSU K T, et al. A green, simple and cost-effective approach to synthesize high quality graphene by electrochemical exfoliation via process optimization[J]. RSC Advances, 2015, 5(67): 54762-54768. |
| [56] |
LI Y F, CHEN S M, LAI W H, et al. Superhydrophilic graphite surfaces and water-dispersible graphite colloids by electrochemical exfoliation[J]. Journal of Chemical Physics, 2013, 139(6): 064703-064714. |
| [57] |
PARVEEN N, ANSARI M O, CHO M H. Simple route for gram synthesis of less defective few layered graphene and its electrochemical performance[J]. RSC Advances, 2015, 5(56): 44920-44927. |
| [58] |
SAHOO S K, MALLIK A. Simple, fast and cost-effective electrochemical synthesis of few layer graphene nanosheets[J]. Nano, 2015, 10(2): 1550019-1550029. |
| [59] |
SAHOO S K, MALLIK A. Synthesis and characterization of conductive few layered graphene nanosheets using an anionic electrochemical intercalation and exfoliation technique[J]. Journal of Materials Chemistry, 2015, 3(41): 10870-10878. |
| [60] |
GURZEDA B, FLORCZAK P, KEMPINSKI M, et al. Synthesis of graphite oxide by electrochemical oxidation in aqueous perchloric acid[J]. Carbon, 2016, 100(1): 540-545. |
| [61] |
PEI S, WEI Q, HUANG K, et al. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation[J]. Nat Commun, 2018, 9(1): 145-154. |
| [62] |
裴嵩峰, 任文才, 黄坤等.一种连续化制备氧化石墨烯微片的方法: ZL201610167450.9[P].2019-05-10. PEI S F, REN W C, HUANG K, et al. A method for continuous preparation of graphene oxide microchips: ZL201610167450.9[P].2019-05-10. |
| [63] |
JUNG S M, MAFRA D L, LIN C T, et al. Controlled porous structures of graphene aerogels and their effect on supercapacitor performance[J]. Nanoscale, 2015, 7(10): 4386-4393. |
| [64] |
WEI W, WANG G, YANG S, et al. Efficient coupling of nanoparticles to electrochemically exfoliated graphene[J]. Journal of the American Chemical Society, 2015, 137(16): 5576-5581. |
| [65] |
LIU Z, ZHANG H, EREDIA M, et al. Water-dispersed high-quality graphene:a green solution for efficient energy storage applications[J]. ACS Nano, 2019, 13(8): 9431-9441. |
| [66] |
LIU Z, PARVEZ K, LI R, et al. Transparent conductive electrodes from graphene/PEDOT:PSS hybrid inks for ultrathin organic photodetectors[J]. Advanced Materials, 2015, 27(4): 669-675. |
| [67] |
MUKHERJEE M D, DHAND C, DWIVEDI N, et al. Facile synthesis of 2-dimensional transparent graphene flakes for nucleic acid detection[J]. Sensors and Actuators B:Chemical, 2015, 210(4): 281-289. |
| [68] |
WANG J, MANGA K K, BAO Q, et al. High-yield synthesis of few-layer graphene flakes through electrochemical expansion of graphite in propylene carbonate electrolyte[J]. Journal of the American Chemical Society, 2011, 133(23): 8888-8891. |
| [69] |
XIA Z Y, GIAMBASTIANI G, CHRISTODOULOU C, et al. Synergic exfoliation of graphene with organic molecules and inorganic ions for the electrochemical production of flexible electrodes[J]. Chem Plus Chem, 2014, 79(3): 439-446. |
| [70] |
ZHOU M, TANG J, CHENG Q, et al. Few-layer graphene obtained by electrochemical exfoliation of graphite cathode[J]. Chemical Physics Letters, 2013, 572(5): 61-65. |
| [71] |
YANG Y, JI X, YANG X, et al. Electrochemically triggered graphene sheets through cathodic exfoliation for lithium ion batteries anodes[J]. RSC Advances, 2013, 3(36): 16130-16135. |
| [72] |
LEROUX Y R, BERGAMINI J F, ABABOU S, et al. Synthesis of functionalized few-layer grapheme through fast electrochemical expansion of graphite[J]. Journal of Electroanalytical Chemistry, 2015, 753(9): 42-46. |
| [73] |
ZOU Y, WANG Y. Interconnecting carbon fibers with the in situ electrochemically exfoliated graphene as advanced binder-free electrode materials for flexible supercapacitor[J]. Scientific Reports, 2015, 5(7): 11792-11798. DOI:10.1038/srep11792 |
| [74] |
GARCÍA-DALÍ S, PAREDES J I, MUNUERA J M. An aqueous cathodic delamination route towards high quality graphene flakes for oil sorption and electrochemical charge storage applications[J]. Chemical Engineering Journal, 2019, 372(9): 226-1239. |
| [75] |
COOPER A J, WILSON N R, KINLOCH I A, et al. Single stage electrochemical exfoliation method for the production of few-layer graphene via intercalation of tetraalkylammonium cations[J]. Carbon, 2014, 66(3): 340-350. |
| [76] |
LU J, YANG J X, WANG J Z, et al. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids[J]. ACS Nano, 2009, 3(8): 2367-2375. |
| [77] |
MAO M, WANG M, HU J, et al. Simultaneous electrochemical synthesis of few-layer graphene flakes on both electrodes in protic ionic liquids[J]. Chemical Communications, 2013, 49(46): 5301-5303. |
| [78] |
NAJAFABADI A T, GYENGE E. High-yield graphene production by electrochemical exfoliation of graphite:novel ionic liquid (IL)-acetonitrile electrolyte with low IL content[J]. Carbon, 2014, 71(5): 58-69. |
| [79] |
LIU F, WANG C J, SUI X, et al. Synthesis of graphene materials by electrochemical exfoliation:recent progress and future potential[J]. Carbon Energy, 2019, 2(9): 1-27. |
| [80] |
WANG S, ZHANG Y, ABIDI N, et al. Wettability and surface free energy of graphene films[J]. Langmuir, 2009, 25(18): 11078-11081. |
| [81] |
ABDELKADER A M, KINLOCH I A, DRYFE R. Continuous electrochemical exfoliation of micrometer-sized graphene using synergistic ion intercalations and organic solvents[J]. ACS Applied Materials & Interfaces, 2014, 6(3): 1632-1639. |
| [82] |
HUANG H, XIA Y, TAO X, et al. Highly efficient electrolytic exfoliation of graphite into graphene sheets based on Li ions intercalation-expansion-microexplosion mechanism[J]. Journal of Materials Chemistry, 2012, 22(21): 10452-10456. |
| [83] |
ZHANG W, ZENG Y, XIAO N, et al. One-step electrochemical preparation of graphene-based heterostructures for Li storage[J]. Journal of Materials Chemistry, 2012, 22(17): 8455-8461. |
| [84] |
GEE C M, TSENG C C, WU F Y, et al. Flexible transparent electrodes made of electrochemically exfoliated graphene sheets from low-cost graphite pieces[J]. Displays, 2013, 34(4): 315-319. |
| [85] |
RYU S H, KIM S, KIM H, et al. Highly conductive polymer composites incorporated with electrochemically exfoliated graphene fillers[J]. RSC Advances, 2015, 5(46): 36456-36460. |
| [86] |
SHINDE D, BRENKER J, EASTON C D, et al. Shear assisted electrochemical exfoliation of graphite to graphene[J]. Langmuir, 2016, 32(14): 3552-3559. |
| [87] |
AFKHAM M, ANUPAM S. Bilayer-rich graphene suspension from electrochemical exfoliation of graphite[J]. Materials & Design, 2018, 156(6): 62-70. |
| [88] |
AFKHAM M, SINGH D K, ANUPAM S. Size distribution of trilayer graphene flakes obtained by electrochemical exfoliation of graphite:effect of the synthesis parameters[J]. Materials Chemistry and Physics, 2018, 220(12): 87-97. |
| [89] |
BAKHSHANDEH R, SHAFIEKHANI A. Ultrasonic waves and temperature effects on graphene structure fabricated by electrochemical exfoliation method[J]. Materials Chemistry and Physics, 2018, 212(6): 95-102. DOI:10.4172/2324-8777-C8-041 |
| [90] |
CHEN C W, LIU Z T, ZHANG Y Z, et al. Sonoelectrochemical intercalation and exfoliation for the preparation of defective graphene sheets and their application as nonenzymatic H2O2 sensors and oxygen reduction catalysts[J]. RSC Advances, 2015, 5(28): 21988-21998. |
| [91] |
THANH D V, OANH P T, HUONG D T, et al. Ultrasonic-assisted cathodic electrochemical discharge for graphene synthesis[J]. Ultrasonics Sonochemistry, 2017, 34(1): 978-983. |
| [92] |
WU L, LI W, LI P, et al. Powder, paper and foam of few-layer graphene prepared in high yield by electrochemical intercalation exfoliation of expanded graphite[J]. Small, 2014, 10(7): 1421-1429. |
| [93] |
MARKOVIC Z M, BUDIMIR M D, KEPIC D P, et al. Semi-transparent, conductive thin films of electrochemical exfoliated graphene[J]. RSC Advances, 2016, 6(45): 39275-39283. |
| [94] |
LIU J, YANG H, ZHEN S G, et al. A green approach to the synthesis of high-quality graphene oxide flakes via electrochemical exfoliation of pencil core[J]. RSC Advances, 2013, 3(29): 11745-11750. DOI:10.1039/c3ra41366g |
| [95] |
LIU J, POH C K, ZHAN D, et al. Improved synthesis of graphene flakes from the multiple electrochemical exfoliation of graphite rod[J]. Nano Energy, 2013, 2(3): 377-386. |
| [96] |
ZHANG Y, XU Y. Simultaneous Electrochemical dual-electrode exfoliation of graphite toward scalable production of high-quality graphene[J]. Advanced Functional Materials, 2019, 29(37): 1902171-1902185. DOI:10.1002/adfm.201902171 |
| [97] |
ACHEE T C, SUN W M, HOPE J T, et al. High-yield scalable graphene nanosheet production from compressed graphite using electrochemical exfoliation[J]. Scientific Reports, 2018, 8(1): 14525-14533. DOI:10.1038/s41598-018-32741-3 |
| [98] |
KUILA T, KHANRA P, KIM N H, et al. Effects of sodium hydroxide on the yield and electrochemical performance of sulfonated poly (ether-ether-ketone) functionalized graphene[J]. Journal of Materials Chemistry:A, 2013, 1(32): 9294-9302. |
| [99] |
OUHIB F, AQIL A, THOMASSIN J M, et al. A facile and fast electrochemical route to produce functional few-layer graphene sheets for lithium battery anode application[J]. Journal of Materials Chemistry:A, 2014, 2(37): 15298-15302. |
| [100] |
ZHANG L, HUANG H, YIN H, et al. Sulfur synchronously electrodeposited onto exfoliated graphene sheets as a cathode material for advanced lithium-sulfur batteries[J]. Journal of Materials Chemistry:A, 2015, 3(32): 16513-16519. |
| [101] |
GU S Y, HSIEH C T, YUAN J Y, et al. Amino-functionalization of graphene nanosheets by electrochemical exfoliation technique[J]. Diamond and Related Materials, 2018, 87(5): 99-106. |
| [102] |
YANG Y, SHI W, ZHANG R, et al. Electrochemical exfoliation of graphite into nitrogen-doped graphene in glycine solution and its energy storage properties[J]. Electrochimica Acta, 2016, 204(6): 100-107. |
| [103] |
LU A K, LI H Y, YU Y. Holey graphene synthesized by electrochemical exfoliation for high-performance flexible microsupercapacitors[J]. Journal of Materials Chemistry:A, 2019, 7(13): 7852-7858. |
| [104] |
ZHOU F, HUANG H, XIAO C, et al. Electrochemically scalable production of fluorine-modified graphene for flexible and high-energy ionogel-based microsupercapacitors[J]. Journal of the American Chemical Society, 2018, 140(26): 8198-8205. |
| [105] |
XIA Z Y, PEZZINI S, TREOSSI E, et al. The exfoliation of graphene in liquids by electrochemical, chemical, and sonication-assisted techniques:a nanoscale study[J]. Advanced Functional Materials, 2013, 23(37): 4684-4693. DOI:10.1002/adfm.201203686 |
| [106] |
MUNUERA J M, PAREDES J I, VILLAR-RODIL S, et al. High quality, low oxygen content and biocompatible graphene nanosheets obtained by anodic exfoliation of different graphite types[J]. Carbon, 2015, 94(11): 729-739. |
| [107] |
HOFMANN M, CHIANG W Y, NGUY N D T, et al. Controlling the properties of graphene produced by electrochemical exfoliation[J]. Nanotechnology, 2015, 26(33): 335607. |
 2020, Vol. 48
2020, Vol. 48